Statistical Thermodynamics of Ideal Gases

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
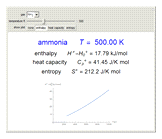
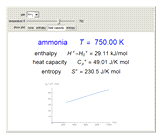
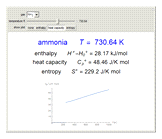
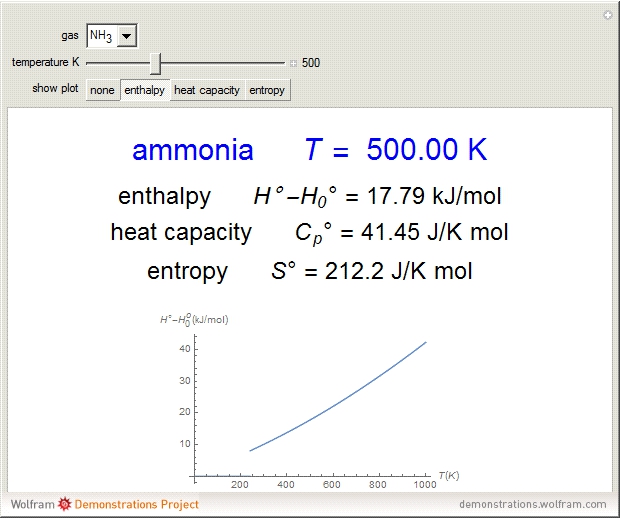
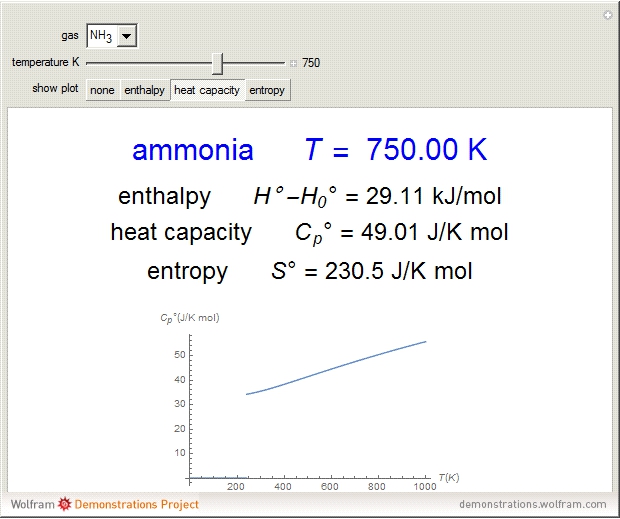
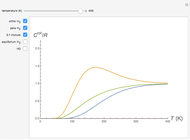
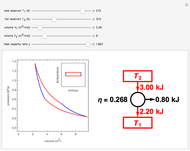
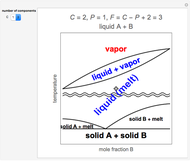
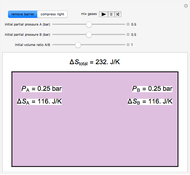
Thermodynamic properties of ideal gases, well approximated by real gases at a pressure of 1 atm, can be computed from molecular and spectroscopic parameters by the methods of statistical mechanics. For monatomic gases, only the molecular weights are needed. For polyatomic molecules, the fundamental vibrational frequencies  and rotational constants
and rotational constants  , and
, and  , usually expressed in wavenumbers (
, usually expressed in wavenumbers ( ), are also required. The rotational symmetry number
), are also required. The rotational symmetry number  is also needed, as well as the electronic degeneracy
is also needed, as well as the electronic degeneracy  for the few molecules with non-singlet electronic ground states, such as
for the few molecules with non-singlet electronic ground states, such as  and NO.
and NO.
Contributed by: S. M. Blinder (March 2009)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference: S. M. Blinder, Advanced Physical Chemistry, London: Macmillan, 1969 pp. 438 ff.
Permanent Citation