Dissolving a Solute

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
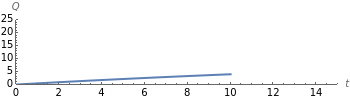
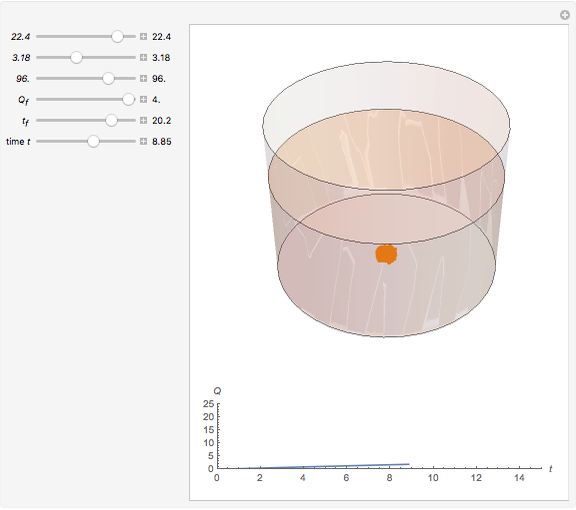
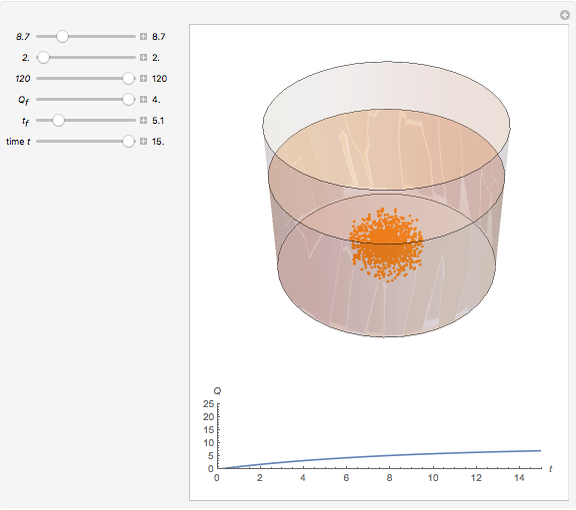
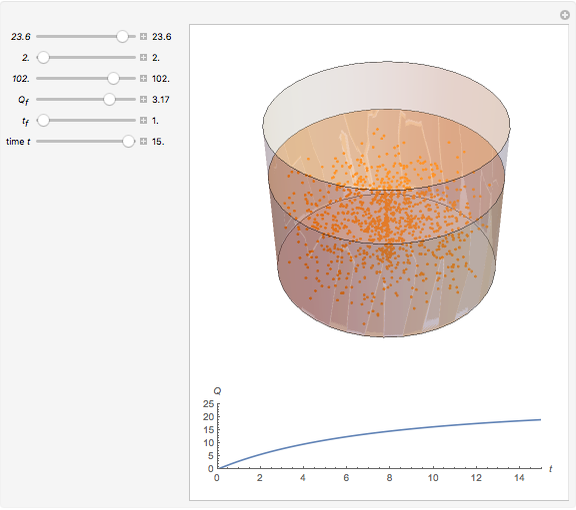
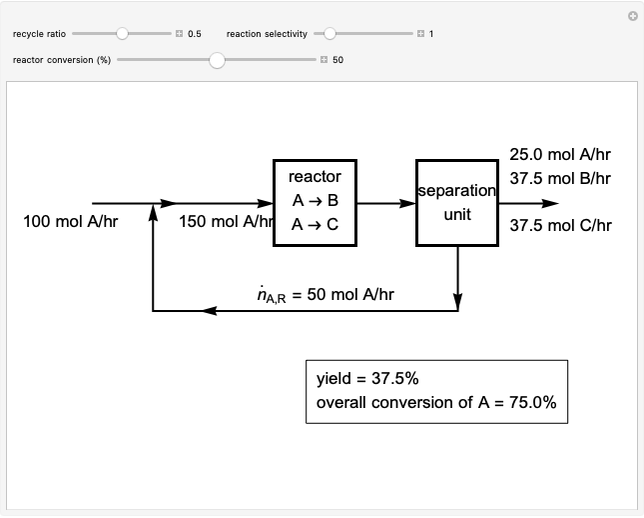
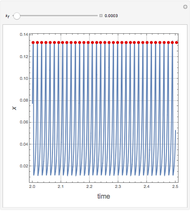
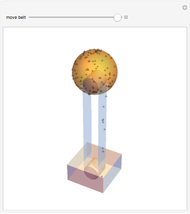
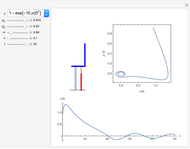
Dissolving of a solid substance in a liquid solvent as a function of time  is proportional to two factors: (1) the difference between the concentration and the saturation concentration of the substance and (2) the quantity of undissolved solid in the solvent. Here the saturation concentration of the substance is taken to be 1 part of solute in
is proportional to two factors: (1) the difference between the concentration and the saturation concentration of the substance and (2) the quantity of undissolved solid in the solvent. Here the saturation concentration of the substance is taken to be 1 part of solute in  parts of solvent. The variables are the mass
parts of solvent. The variables are the mass  of solute, the mass
of solute, the mass 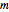 of solvent, the time to saturation
of solvent, the time to saturation 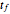 , and the observed concentration
, and the observed concentration 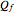 .
.
Contributed by: Enrique Zeleny (February 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The relevant differential equation is
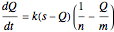
with solution
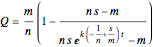 .
.
The constant  can be determined from
can be determined from 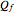 and
and 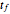 .
.
Reference
[1] C. R. Wylie and L. C. Barrett, Advanced Engineering Mathematics, 4th ed., New York: McGraw-Hill, 1975 pp. 26-27.
Permanent Citation