Diffusion-Controlled Evaporation of an Aerosol Droplet

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
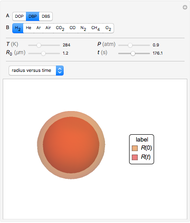
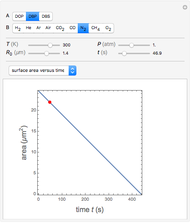
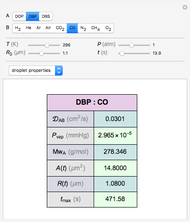
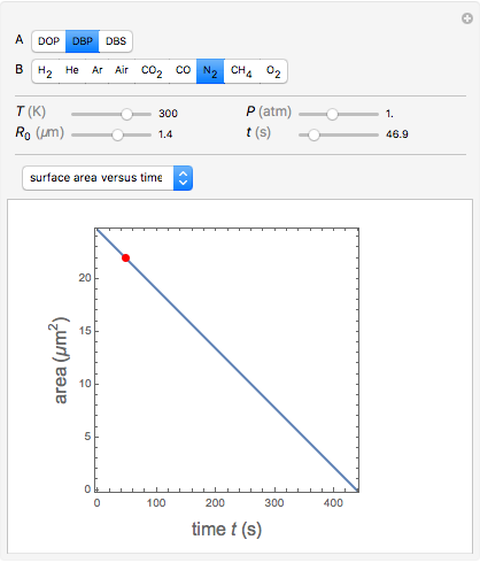
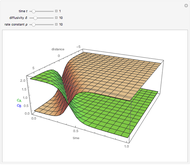
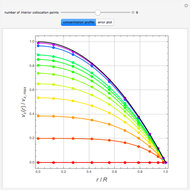
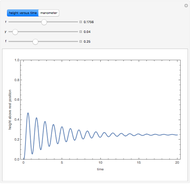
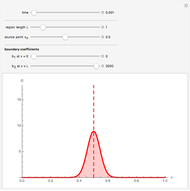
Diffusion-controlled evaporation of aerosol droplets in an ambient gas is important in various engineering and scientific fields, such as premixing air and fuel, ink-jet printing, spraying of pesticides, biochemical assays, and atmospheric pollution. For isothermal, quasi-steady evaporation, Maxwell [1], showed that the time rate of change of the droplet radius  is given by
is given by
Contributed by: Brian G. Higgins and Housam Binous (February 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] J. C. Maxwell, The Scientific Papers of James Clerk Maxwell, Vol. II, (W. D. Niven, ed.), Cambridge, England: Cambridge University Press, 1890 p. 625. archive.org/details/scientificpapers02maxwuoft.
[2] R. B. Bird, W. E. Stewart, and E. N. Lightfoot, Transport Phenomena, 2nd ed., New York: John Wiley & Sons, 2002.
[3] A. K. Ray, E. J. Davis, and P. Ravindran, "Determination of Ultra-Low Vapor Pressures by Submicron Droplet Evaporation," Journal of Chemical Physics 71(2), 1979 pp. 582–587. doi:10.1063/1.438408.
Permanent Citation