Driving a Reaction by Chemical Coupling

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
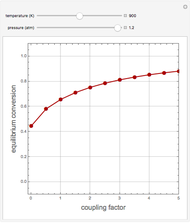
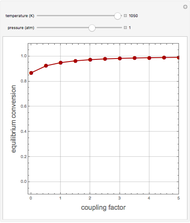
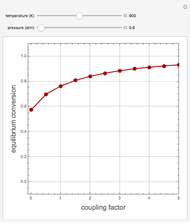
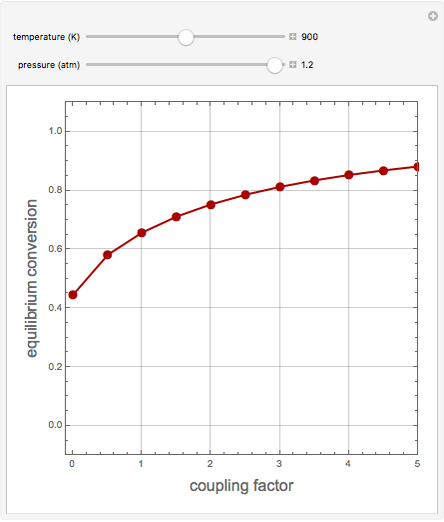
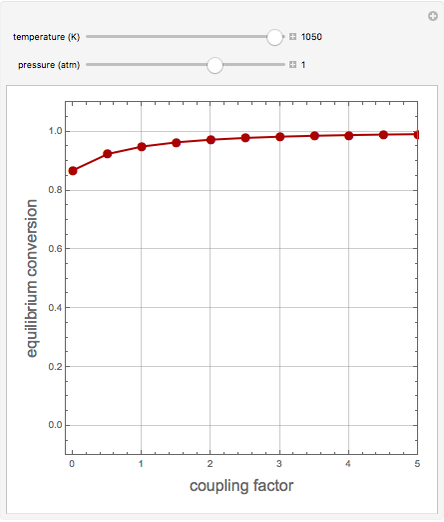
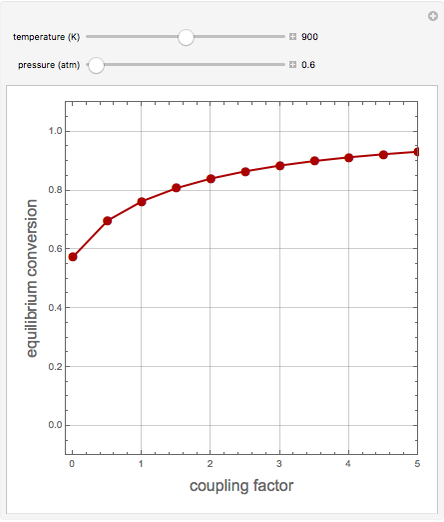
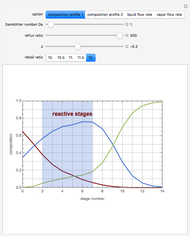
1,3-Butadiene can be produced by the gas-phase catalytic dehydrogenation of 1-Butene by the reaction  . To suppress the reverse reaction, an inert gas (e.g. steam) can be added to the feed stream of the reactor. Instead, we choose here to add
. To suppress the reverse reaction, an inert gas (e.g. steam) can be added to the feed stream of the reactor. Instead, we choose here to add  to the reactor. Assume a suitable catalyst is present for the water-gas shift reaction
to the reactor. Assume a suitable catalyst is present for the water-gas shift reaction  ; this second reaction consumes
; this second reaction consumes  . Therefore, in accordance with Le Chatelier's principle, higher conversion for the dehydrogenation should be achieved. This Demonstration illustrates how one can drive a chemical reaction (i.e. the dehydrogenation reaction) by coupling to a second reaction.
. Therefore, in accordance with Le Chatelier's principle, higher conversion for the dehydrogenation should be achieved. This Demonstration illustrates how one can drive a chemical reaction (i.e. the dehydrogenation reaction) by coupling to a second reaction.
Contributed by: Housam Binous, Mohammad Mozahar Hossain, and Ahmed Bellagi (January 2016)
(King Fahd University of Petroleum & Minerals, KSA; ENIM, University of Monastir, Tunisia)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] J. R. Elliott and C. T. Lira, Introductory Chemical Engineering Thermodynamics, 2nd ed., Englewood Cliffs, NJ: Prentice Hall International Editions, 2012.
Permanent Citation