Gibbs Free Energy Minimization Applied to the Haber Process

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
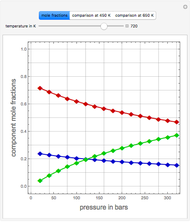
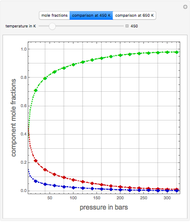
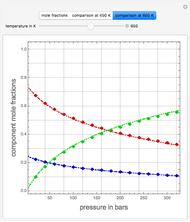
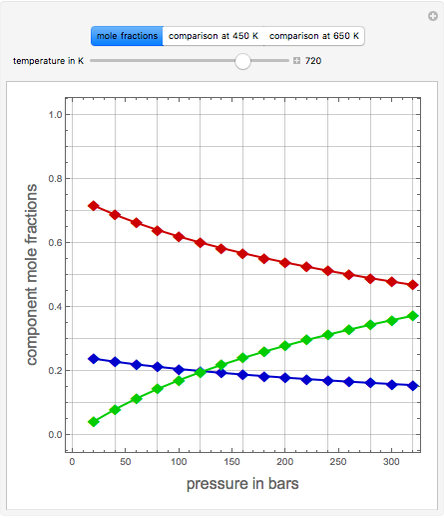
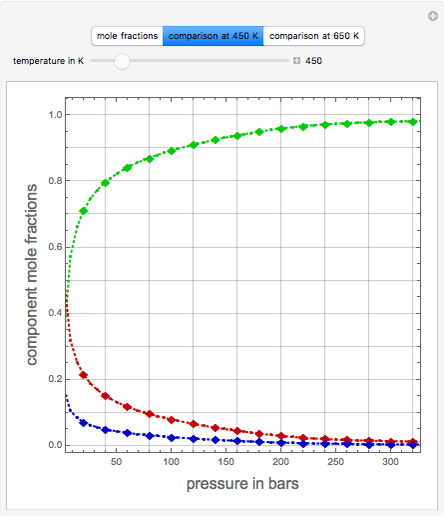
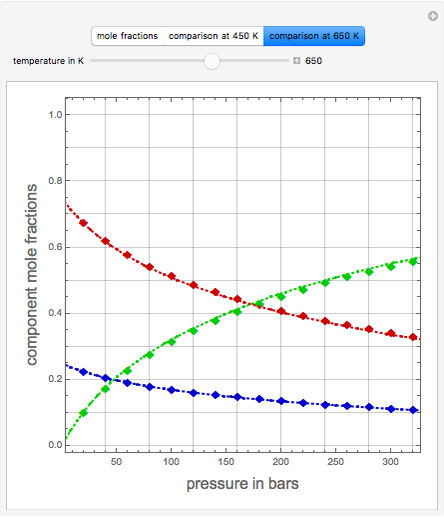
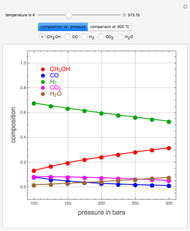
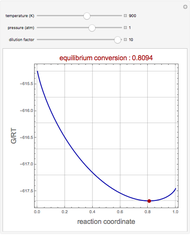
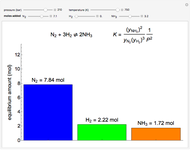
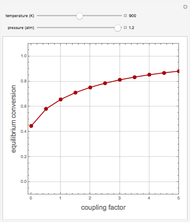
Consider the high-pressure synthesis of ammonia ( ), known as the Haber process. This Demonstration shows plots of the mole fractions of all three components (
), known as the Haber process. This Demonstration shows plots of the mole fractions of all three components ( ,
,  , and
, and  , in red, blue, and green, respectively) for temperatures ranging from 420 K to 800 K.
, in red, blue, and green, respectively) for temperatures ranging from 420 K to 800 K.
Contributed by: Housam Binous, Ahmed Bellagi, Brian G. Higgins, Ahmed Aheed, and Mohammad Mozahar Hossain (January 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] I. Barin and G. Platzki, Thermochemical Data of Pure Substances, 3rd ed., New York: VCH Publishers, Inc., 1995.
[2] S. I. Sandler, Chemical and Engineering Thermodynamics, 3rd ed., New York: John Wiley & Sons, 1999.
[3] H. Binous, A. Aheed and M. M. Hossain, "Haber Process and Steam-Coal Gasification: Two Standard Thermodynamic Problems Elucidated Using Two Distinct Approaches," Computer Applications in Engineering Education Journal, DOI: 10.1002/cae.21672.
Permanent Citation
"Gibbs Free Energy Minimization Applied to the Haber Process"
http://demonstrations.wolfram.com/GibbsFreeEnergyMinimizationAppliedToTheHaberProcess/
Wolfram Demonstrations Project
Published: January 15 2015