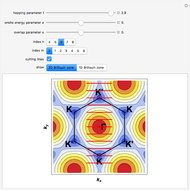
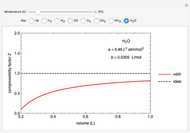
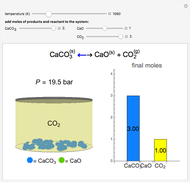
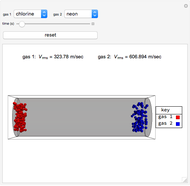
Henry's Law for Oxygen and Carbon Dioxide

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
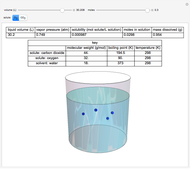
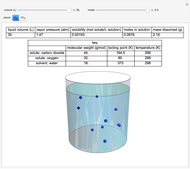
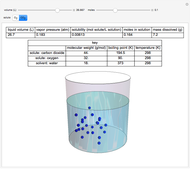
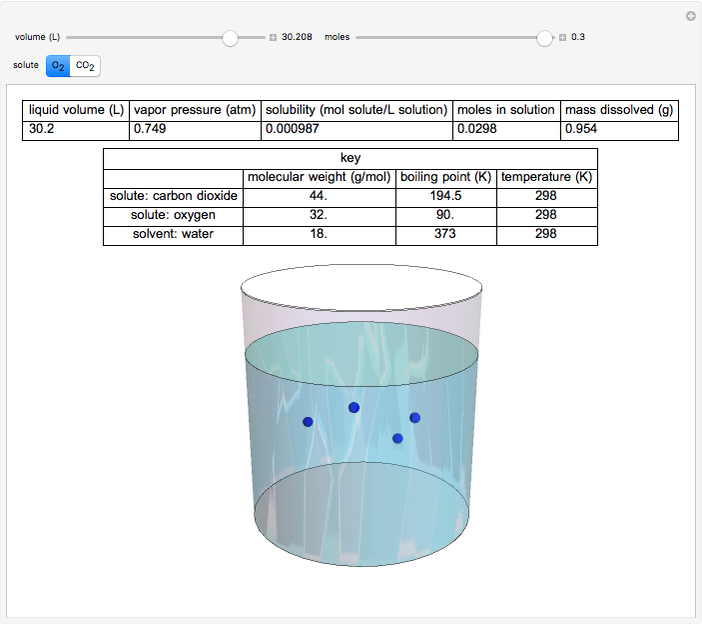
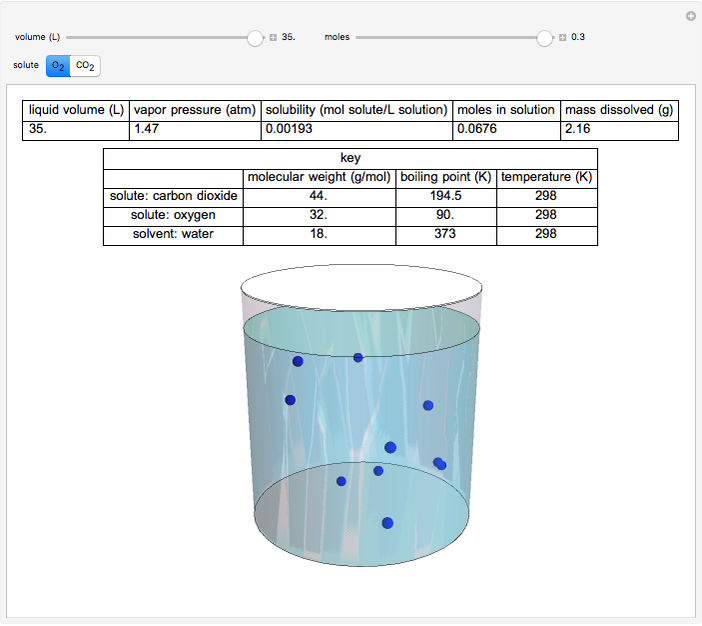
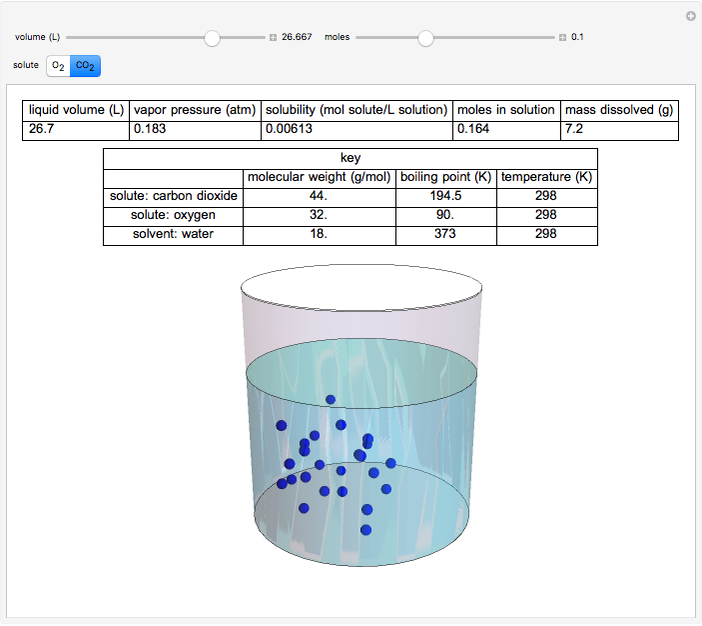
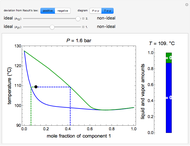
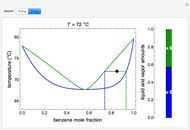
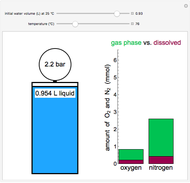
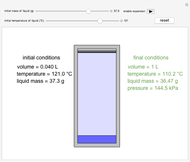
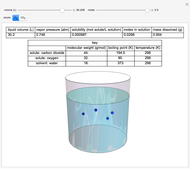
This Demonstration considers the solubility of two different gases,  and
and  , in water. The "volume (L)" slider changes the amount of water in the cylinder, while the "moles" slider changes the number of moles of gas in the cylinder, roughly modeled by the number of blue spheres. By Henry's law,
, in water. The "volume (L)" slider changes the amount of water in the cylinder, while the "moles" slider changes the number of moles of gas in the cylinder, roughly modeled by the number of blue spheres. By Henry's law,  , the solubility of a gas, is proportional to
, the solubility of a gas, is proportional to  , its partial pressure above the solution:
, its partial pressure above the solution:  . At 298 K, the Henry's law constant
. At 298 K, the Henry's law constant  equals
equals  for
for  and
and  for
for  .
.
Contributed by: Farris Jaamour and Chris Fredricks (April 2017)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
Reference
[1] W. Emmerich, B. Rubin and R. J. Wilcock, "Low-Pressure Solubility of Gases in Liquid Water," Chemical Reviews, 77(2), 1977 pp. 219–262.
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation