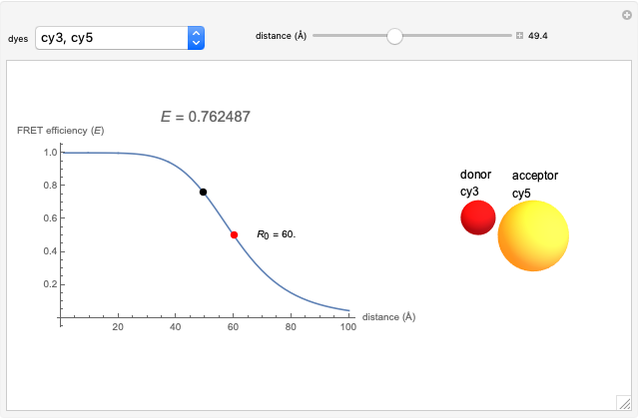
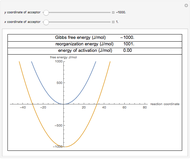
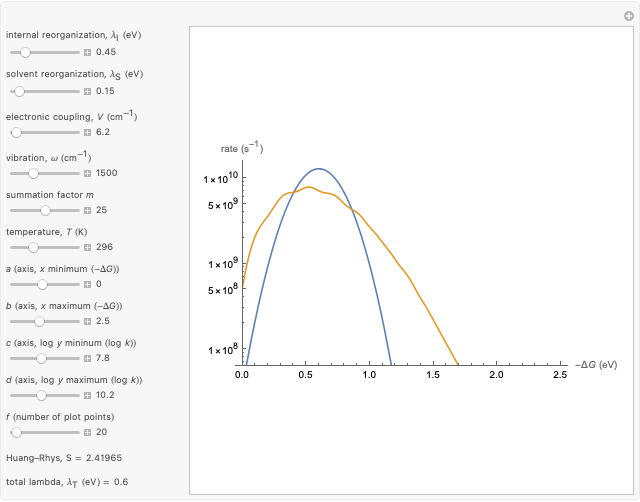
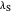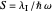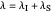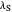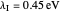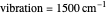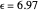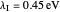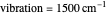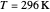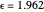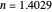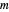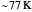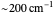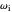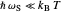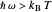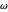Marcus Theory of Electron Transfer 5: Two-Dimensional Bell-Shaped Marcus Curves

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
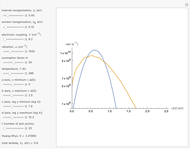
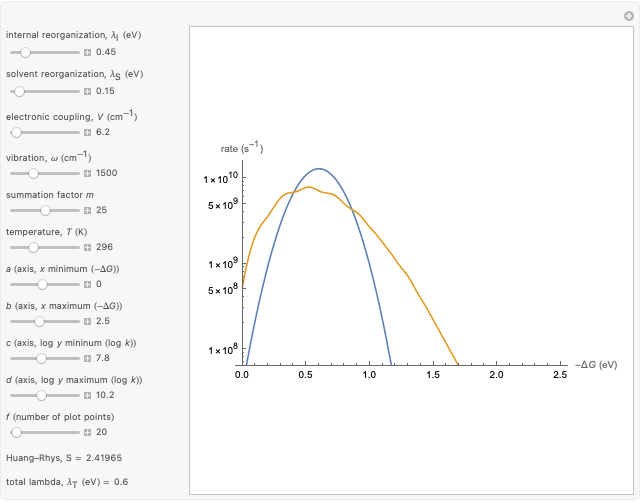
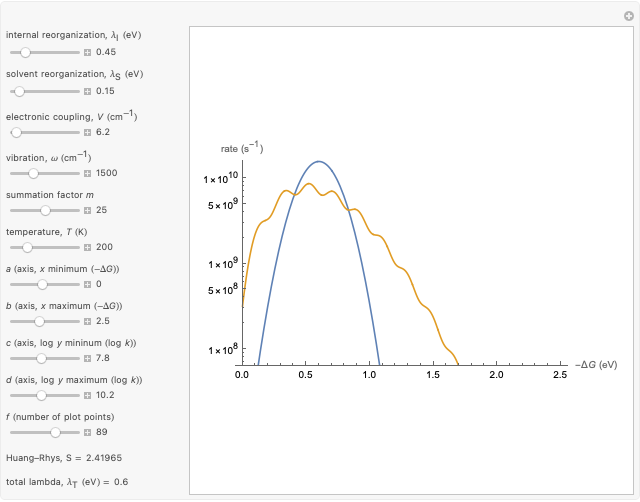
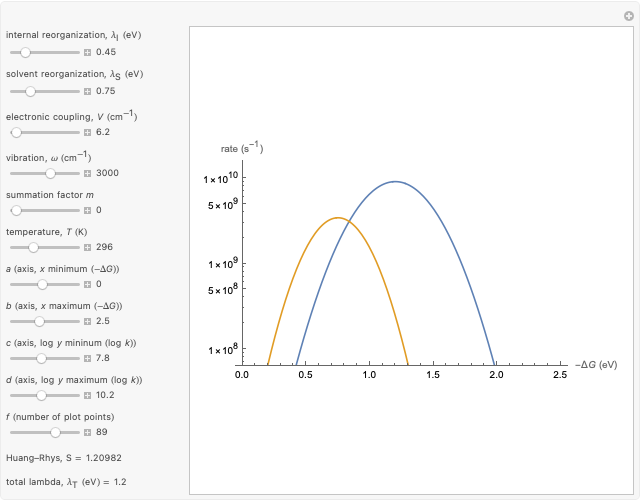
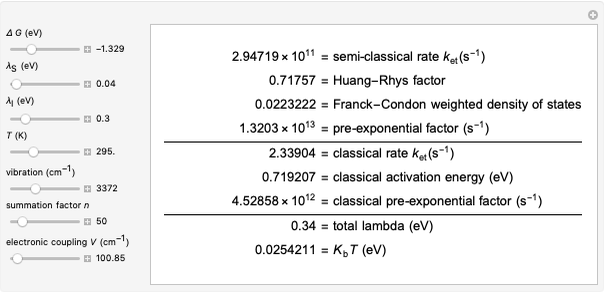
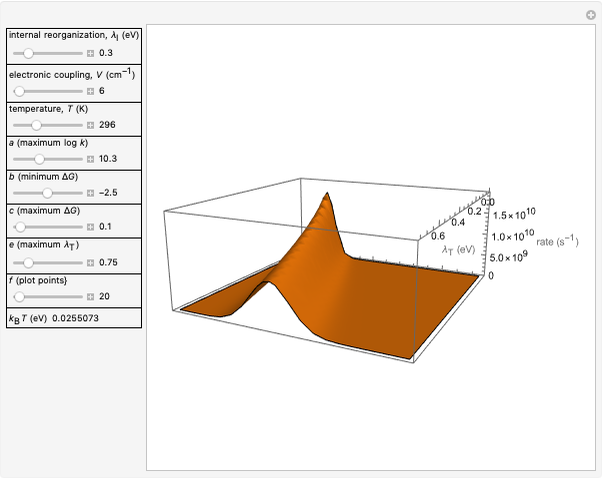
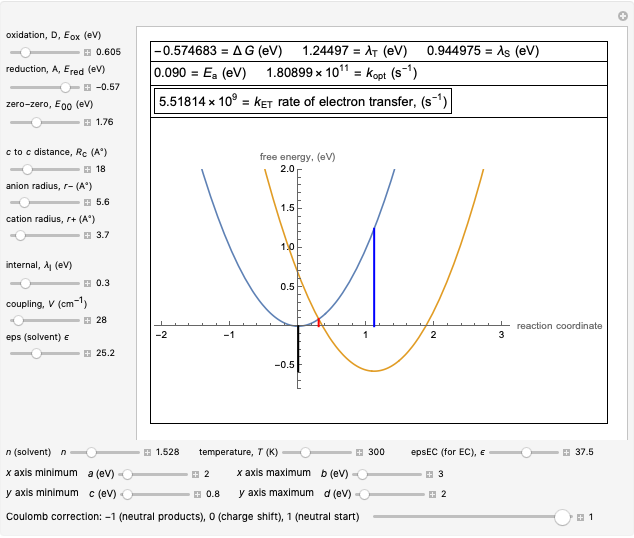
This Demonstration compares the two-dimensional bell-shaped Marcus curves for the classical (blue) and semiclassical approach (yellow). The main difference, in the Marcus inverted region (at large negative values of  ), is simple to observe and manipulate. Note that we show log
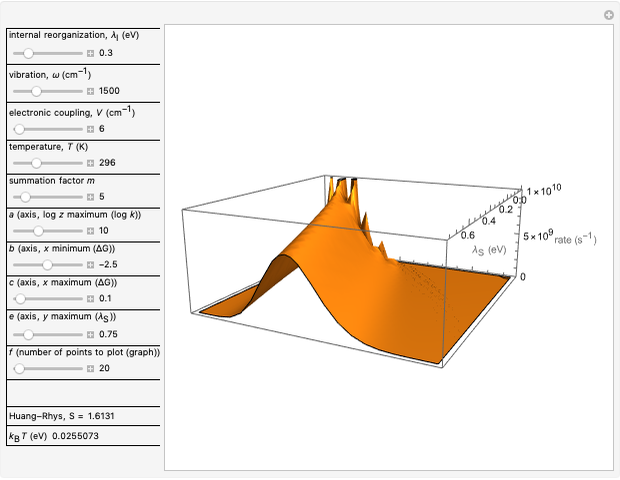
), is simple to observe and manipulate. Note that we show log  plots. The three-dimensional plots in earlier Demonstrations (see Related Links) are linear plots in the rate
plots. The three-dimensional plots in earlier Demonstrations (see Related Links) are linear plots in the rate  . Note that at very low temperatures, both models become inaccurate.
. Note that at very low temperatures, both models become inaccurate.
Contributed by: René M. Williams (January 2023)
Open content licensed under CC BY-NC-SA
Snapshots
Details
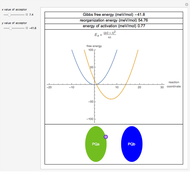
Snapshot 1: data based on the work of Closs and Miller (focused here on the iso-octane data) at 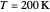 , with low solvent reorganization energies
, with low solvent reorganization energies
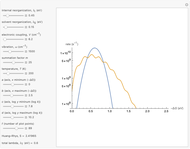
Snapshot 2: for 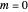 , the semiclassical Marcus equation behaves similarly to the CME
, the semiclassical Marcus equation behaves similarly to the CME
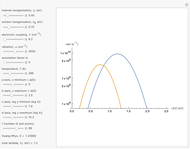
Snapshot 3: in the high temperature limit (1000 K), the semiclassical Marcus equation reduces to the CME
Snapshot 4: the very low temperature graph shows the effects of the summation factor: bell curves are added and shifted with increase of 
Input data based on the work of Closs and Miller (focused here on the iso-octane data) at 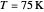 .
.
References
[1] R. A. Marcus, "Electron Transfer Reactions in Chemistry: Theory and Experiment (Nobel Lecture)," Angewandte Chemie International Edition, 32(8), 1993 pp. 1111–1121. doi:10.1002/anie.199311113.
[2] S. Chaudhuri, S. Hedström, D. D. Méndez-Hernández, H. P. Hendrickson, K. A. Jung, J. Ho and V. S. Batista, "Electron Transfer Assisted by Vibronic Coupling from Multiple Modes," Journal of Chemical Theory and Computation, 13(12), 2017 pp. 6000–6009. doi:10.1021/acs.jctc.7b00513.
[3] P. F. Barbara, T. J. Meyer and M. A. Ratner, "Contemporary Issues in Electron Transfer Research," Journal of Physical Chemistry, 100(31), 1996 pp. 13148–13168. doi:10.1021/jp9605663.
[4] G. L. Closs and J. R. Miller, "Intramolecular Long-Distance Electron Transfer in Organic Molecules," Science, 240(4851), 1988 pp. 440–447. doi:10.1126/science.240.4851.440.
[5] P. Hudhomme and R. M. Williams, "Energy and Electron Transfer in Photo- and Electro-active Fullerene Dyads," Handbook of Carbon Nano Materials (F. D'Souza and K. M. Kadish, eds.), Hackensack, NJ: World Scientific, 2011 pp. 545–591. doi:10.1142/9789814327824_0017.
[6] R. M. Williams. "Introduction to Electron Transfer." (Nov 21, 2021) doi:10.13140/RG.2.2.16547.30244.
[7] R. M. Williams. Photoinduced Electron Transfer—The Classical Marcus Theory [Video]. (Nov 21, 2021) youtu.be/YFzeMMOvhl0.
[8] R. M. Williams. Photoinduced Electron Transfer—The Semi-classical Marcus–Levich–Jortner Theory [Video]. (Nov 21, 2021) youtu.be/GnPIbH6nM9o.
[9] R. M. Williams. University of Amsterdam. (Nov 21, 2021) www.uva.nl/en/profile/w/i/r.m.williams/r.m.williams.html.
[10] J. Idé and G. Raos. "ChargeTransport: Charge Transfer Rates and Charge Carrier Mobilities." (Nov 21, 2021) cran.r-project.org/src/contrib/Archive/ChargeTransport.
[11] "What Is R?" The R Foundation. (Nov 21, 2021) www.r-project.org/about.html.
[12] A. Sarai, "Energy-Gap and Temperature Dependence of Electron and Excitation Transfer in Biological Systems," Chemical Physics Letters, 63(2), 1979 pp. 360–366. doi:10.1016/0009-2614(79)87036-0.
[13] J. R. Miller, J. V. Beitz and R. K. Huddleston, "Effect of Free Energy on Rates of Electron Transfer between Molecules," Journal of the American Chemical Society, 106(18), 1984 pp. 5057–5068. doi:10.1021/ja00330a004.
[14] M. R. Gunner, D. E. Robertson and P. L. Dutton, "Kinetic Studies on the Reaction Center Protein from Rhodopseudomonas sphaeroides: The Temperature and Free Energy Dependence of Electron Transfer between Various Quinones in the QA Site and the Oxidized Bacteriochlorophyll Dimer," Journal of Physical Chemistry, 90(16), 1986 pp. 3783–3795. doi:10.1021/j100407a054.
[15] R. Rujkorakarn and F. Tanaka, "Three-Dimensional Representations of Photo-induced Electron Transfer Rates in Pyrene- -N,N'-dimethylaniline Systems Obtained by Three Electron Transfer Theories," Journal of Molecular Graphics and Modelling, 27(5), 2009 pp. 571–577. doi:10.1016/j.jmgm.2008.09.008.
-N,N'-dimethylaniline Systems Obtained by Three Electron Transfer Theories," Journal of Molecular Graphics and Modelling, 27(5), 2009 pp. 571–577. doi:10.1016/j.jmgm.2008.09.008.
[16] T. Unger, S. Wedler, F.-J. Kahle, U. Scherf, H. Bässler and A. Köhler, "The Impact of Driving Force and Temperature on the Electron Transfer in Donor–Acceptor Blend Systems," The Journal of Physical Chemistry C, 121(41), 2017 pp. 22739–22752. doi:10.1021/acs.jpcc.7b09213.
[17] W. W. Parson, "Generalizing the Marcus Equation," The Journal of Chemical Physics, 152(18), 2020 184106. doi:10.1063/5.0007569.
[18] J. B. Kelber, N. A. Panjwani, D. Wu, R. Gómez-Bombarelli, B. W. Lovett, J. J. L. Morton and H. L. Anderson, "Synthesis and Investigation of Donor–Porphyrin–Acceptor Triads with Long-Lived Photo-Induced Charge-Separate States," Chemical Science, 6, 2015 pp. 6468-6481. https://pubs.rsc.org/en/content/articlelanding/2015/sc/c5sc01830g.
[19] G. Lanzani, "Charge Transfer and Transport," The Photophysics behind Photovoltaics and Photonics, Weinheim: Wiley-VCH, 2012 pp. 145–176. doi:10.1002/9783527645138.ch8.
Permanent Citation