Production of Anhydrous Ethanol Using an Extractive Distillation Column

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
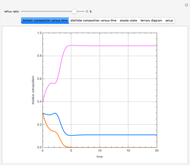
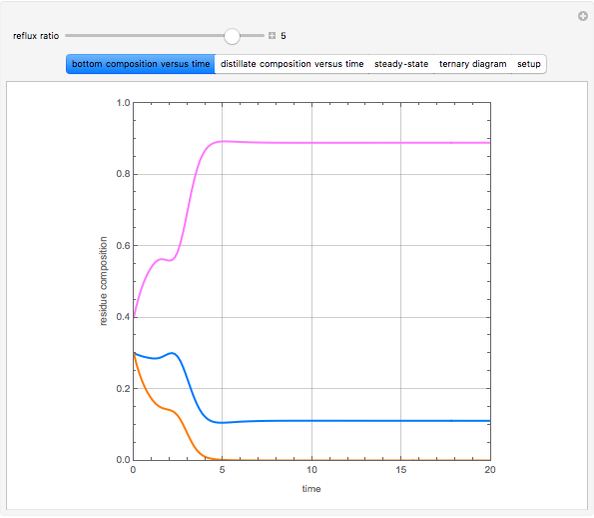
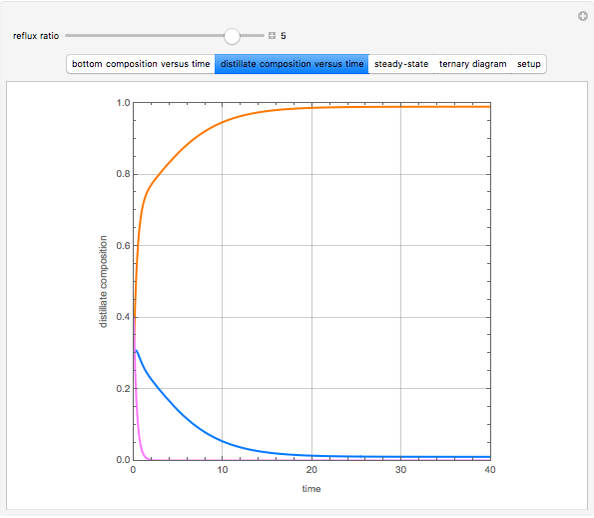
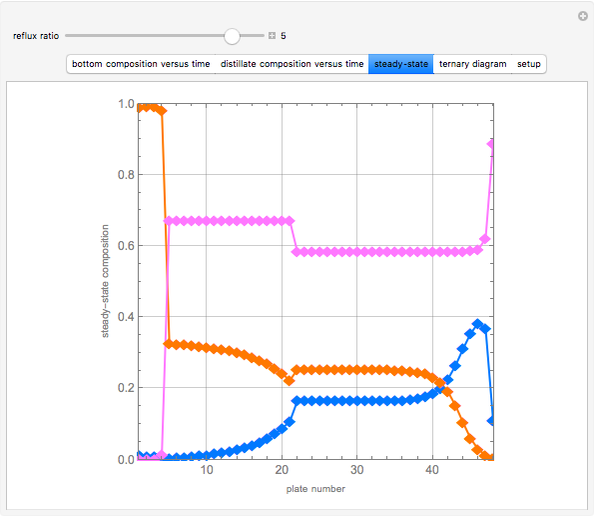
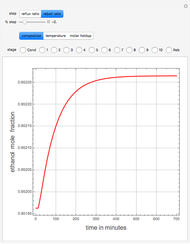
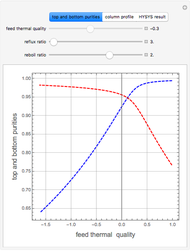
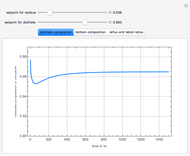
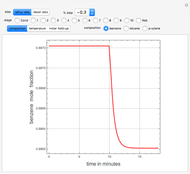
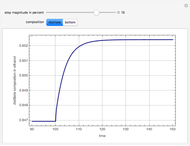
Consider a binary mixture of water and ethanol at 1 atm. This mixture exhibits a positive azeotrope ( ethanol), so that anhydrous ethanol cannot be produced by a simple distillation column. If an entrainer (e.g. ethylene glycol) is used in an extractive distillation column, then one can recover pure ethanol as a distillate stream, as shown in the setup snapshot. The extractive distillation breaks the azeotrope. Water exits the column with ethylene glycol at the bottom and a second column is needed in order to regenerate the entrainer. A make-up stream of ethylene glycol is also required to compensate for the small losses of the entrainer.
ethanol), so that anhydrous ethanol cannot be produced by a simple distillation column. If an entrainer (e.g. ethylene glycol) is used in an extractive distillation column, then one can recover pure ethanol as a distillate stream, as shown in the setup snapshot. The extractive distillation breaks the azeotrope. Water exits the column with ethylene glycol at the bottom and a second column is needed in order to regenerate the entrainer. A make-up stream of ethylene glycol is also required to compensate for the small losses of the entrainer.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation