Rotation-Vibration Energy Level Transitions of a Diatomic Rotor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
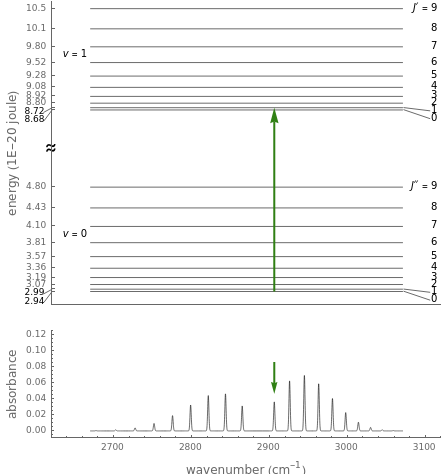
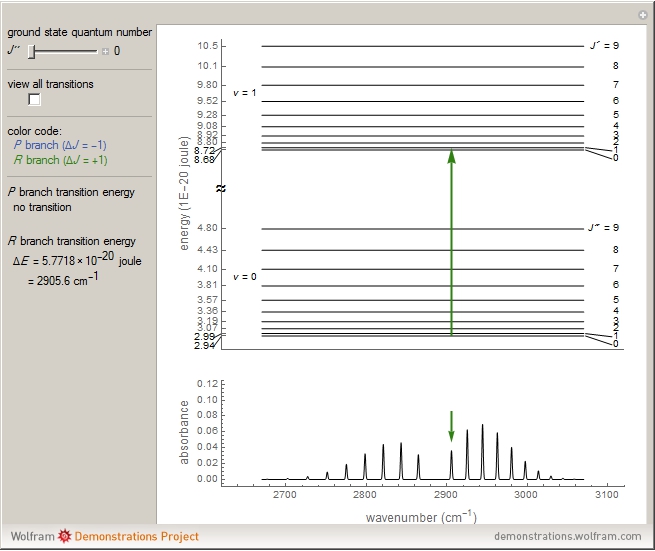
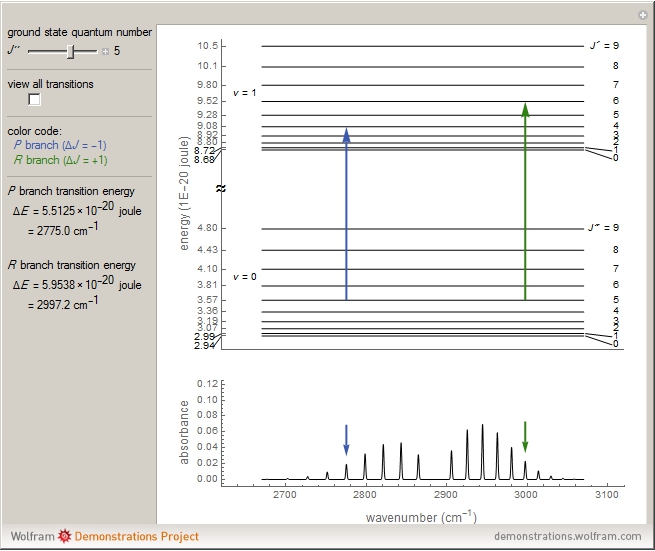
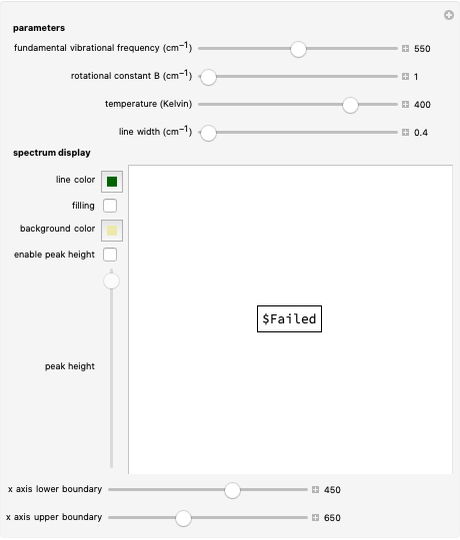
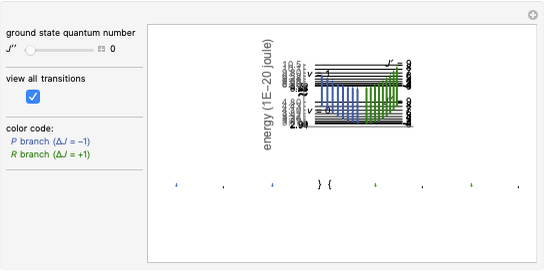
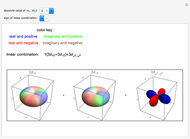
This Demonstration shows the energy level transitions associated with each line observed in a rotationally resolved infrared band spectrum, in which the 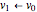 vibrational transition is coupled with
vibrational transition is coupled with  rotational transitions. For a diatomic molecule the vibrational and rotational energy levels are quantized and the selection rules are
rotational transitions. For a diatomic molecule the vibrational and rotational energy levels are quantized and the selection rules are  (vibration) and
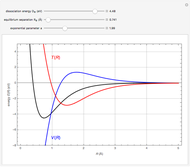
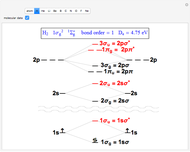
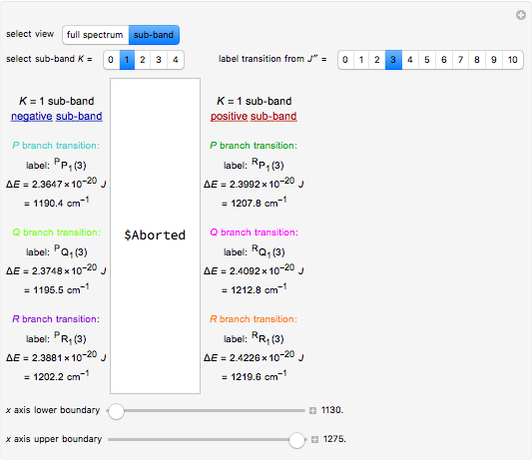
(vibration) and 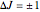 (rotation). Only transitions that meet the selection rule requirements are allowed, and as a result discrete spectral lines are observed, as shown in the bottom graphic. The position of a spectral line corresponds to the energy difference between the initial and final states of the transition. These energy level transitions from the ground to excited rotation-vibration states are shown in the top graphic. The spectrum consists of a
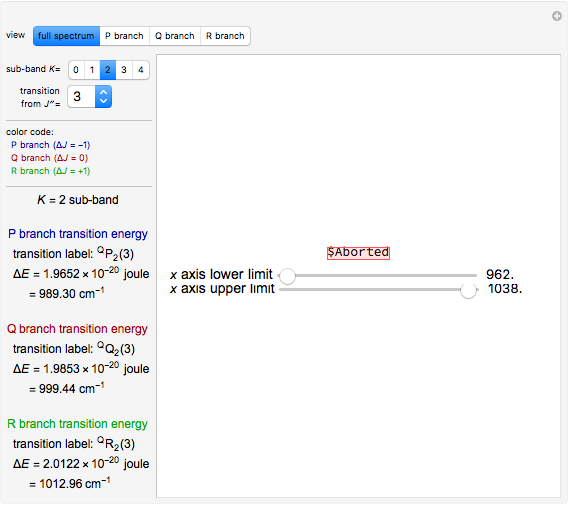
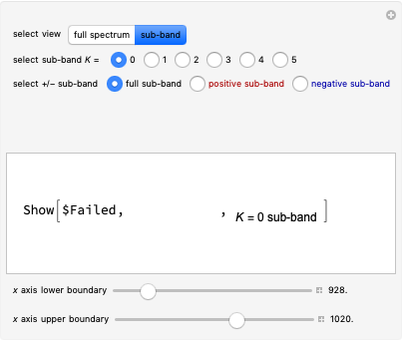
(rotation). Only transitions that meet the selection rule requirements are allowed, and as a result discrete spectral lines are observed, as shown in the bottom graphic. The position of a spectral line corresponds to the energy difference between the initial and final states of the transition. These energy level transitions from the ground to excited rotation-vibration states are shown in the top graphic. The spectrum consists of a  branch (
branch ( ,
,  , smaller wavenumbers, lower energy transitions) and an
, smaller wavenumbers, lower energy transitions) and an 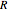 branch (
branch ( ,
,  , larger wavenumbers, higher energy transitions). The central gap between the
, larger wavenumbers, higher energy transitions). The central gap between the  and
and 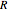 branches represents the forbidden
branches represents the forbidden  branch since the
branch since the 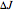 selection rule makes the
selection rule makes the  transitions forbidden. A
transitions forbidden. A  branch can appear, however, if the molecule has electronic angular momentum, a well-known case being the NO molecule.
branch can appear, however, if the molecule has electronic angular momentum, a well-known case being the NO molecule.
Contributed by: Whitney R. Hess and Lisa M. Goss (Idaho State University) (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
This Demonstration utilizes molecular constants of the molecule 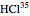 and the infrared spectrum is simulated at a temperature of 200 Kelvin.
and the infrared spectrum is simulated at a temperature of 200 Kelvin.
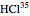 molecular constants:
molecular constants:
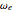 = 2990.946
= 2990.946 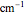
 = 52.8186
= 52.8186 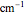
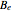 = 10.59341
= 10.59341 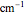
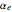 = 0.30718
= 0.30718 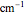
 = 5.3194E-04
= 5.3194E-04 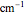
The intensity of the observed spectral lines reflects the dependence on the thermal population of the initial rotational energy levels and the dependence on the quantum number 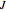 , not on the magnitude of the corresponding transition energy.
, not on the magnitude of the corresponding transition energy.
Note: the  axis on the energy level diagram (top graphic) is arbitrary. The arrows indicating transitions in the top graphic are spread out for clarity and so that their positioning lies directly above the corresponding transition wavenumber in the spectrum below (bottom graphic).
axis on the energy level diagram (top graphic) is arbitrary. The arrows indicating transitions in the top graphic are spread out for clarity and so that their positioning lies directly above the corresponding transition wavenumber in the spectrum below (bottom graphic).
References:
[1] P. Atkins and J. de Paula, Physical Chemistry, New York: Oxford University Press, 2006.
[2] G. Herzberg, Molecular Spectra and Molecular Structure I. Spectra of Diatomic Molecules, Princeton, New Jersey: D. Van Nostrand Company, Inc., 1950.
[3] K. P. Huber and G. Herzberg, Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules, New York: Van Nostrand Reinhold Company, 1979.
[4] Diatomic Constants for HCl. webbook.nist.gov/cgi/cbook.cgi?ID=C7647010&Units=SI&Mask=1000# Diatomic (NIST Chemistry WebBook. webbook.nist.gov/chemistry).
Permanent Citation