Titration of Common Food Acids

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
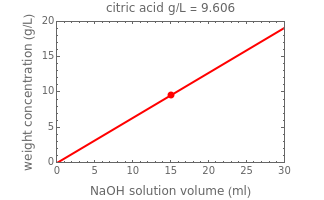
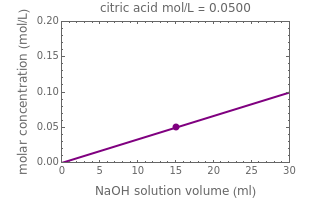
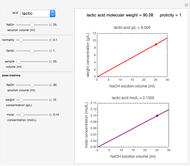
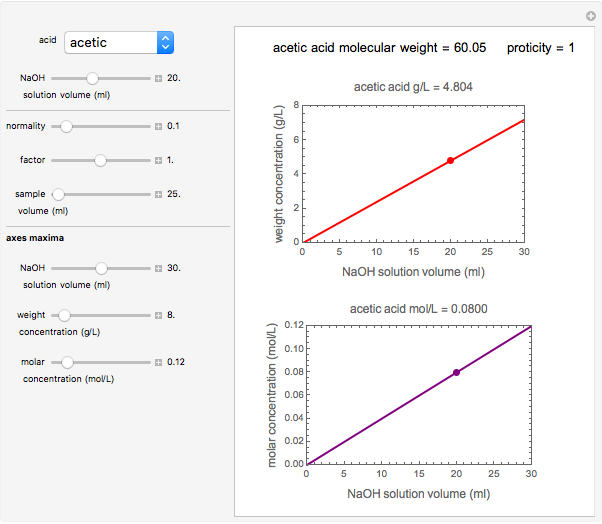
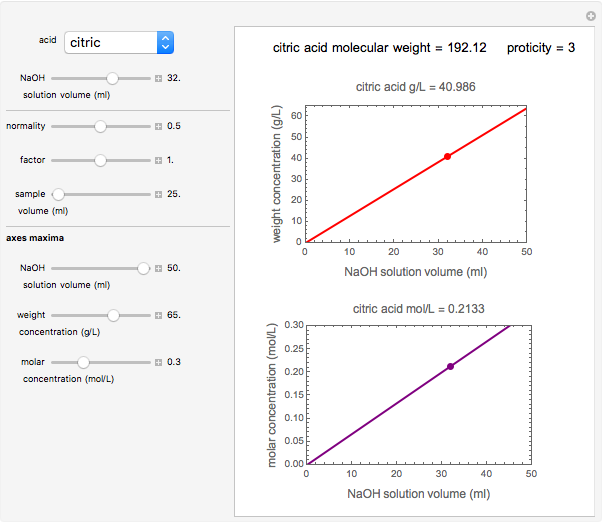
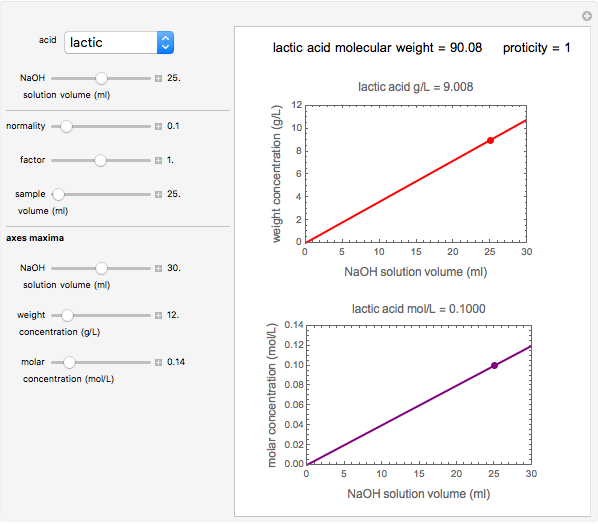
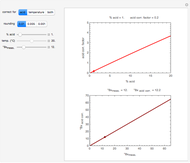
Titration of a given volume of liquid food with a calibrated sodium hydroxide solution is a common method used to determine the food's acid content. The result is expressed in terms of equivalent concentration of a characteristic or dominant acid in the particular food. This Demonstration converts the volume (milliliters) of a sodium hydroxide solution of known strength needed to neutralize six common food acids into their weight per volume and molar concentrations. The six acids are acetic, citric, lactic, malic, phosphoric, and tartaric.
Contributed by: Amy D. Kim, Mark D. Normand, and Micha Peleg (December 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: acetic acid in vinegar
Snapshot 2: citric acid in frozen orange juice concentrate
Snapshot 3: lactic acid in pickles
Snapshot 4: malic acid in apple juice
Snapshot 5: phosphoric acid in a cola beverage
Snapshot 6: tartaric acid in grape juice
In determining acidity by titration, the acid is neutralized by an alkaline solution, most commonly, sodium hydroxide (NaOH). In a monoprotic acid titration, equilibrium is reached at a particular pH, which can be detected with a pH meter or visually by adding an indicator that changes its color at or close to the equilibrium pH. With a biprotic acid, there are two equilibria reflecting the dissociations:
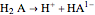 and
and 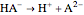 .
.
Thus, the total acid content is determined by titrating to the second equilibrium pH.
In a triprotic acid, there are three equilibria reflecting the dissociations:
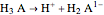 ,
, 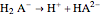 , and
, and 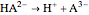 .
.
However, in some cases—phosphoric acid being a notable example—the third equilibrium is reached at such a high pH level that the electrode of the pH meter starts to react with the sodium ions and produces erroneous results. In such a case, the titration is stopped at the first or second equilibrium and the calculation is adjusted accordingly.
In this Demonstration, the acid is chosen by a pop-up menu. The sample volume, the volume of the NaOH solution needed to reach the appropriate pH, the NaOH solution's nominal normality, and its calibration factor are all entered with sliders. The display includes plots of the relationships of the chosen acid's concentration in g/L and molarity as a function of the NaOH solution's volume. A moving point indicates the actual volume. The numerical acid contents, its molecular weight, and proticity are displayed above the plots. The ranges of the plot axes can also be adjusted with sliders.
Almost all foods contain more than a single acid. However, the food's acidity is traditionally calculated and expressed in terms of an equivalent concentration of the dominant acid.
References
[1] W. Horwitz (ed.), Official Methods of Analysis of AOAC International, 17th ed., Gaithersburg, MD: Association of Official Analytical Chemists, 2000.
[2] ChemBuddy. "Determination of Phosphoric Acid Concentration by Acid-Base Titration." (Dec 8, 2014) www.titrations.info/acid-base-titration-phosphoric-acid.
[3] Anon, "Using a pH Titration to Determine the Acid Content of Soft Drinks." 2015. Apr 28, 2015) faculty.ccbcmd.edu/~cyau/124%2010%20Titration%20of%20a%20Cola%20Product%202011.pdf
Permanent Citation