Analogies to Understand Stoichiometry

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
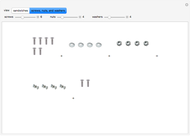
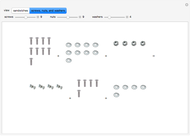
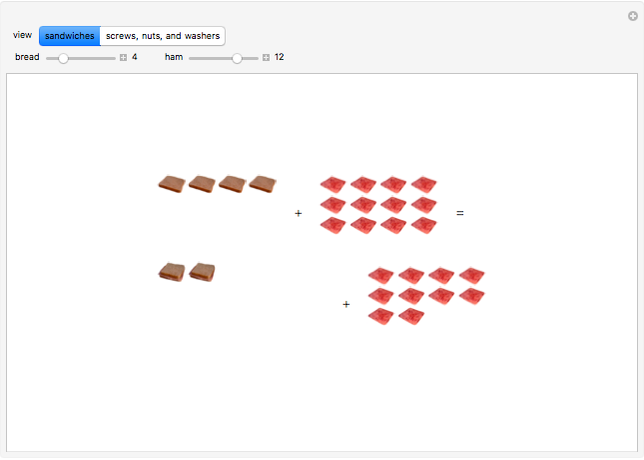
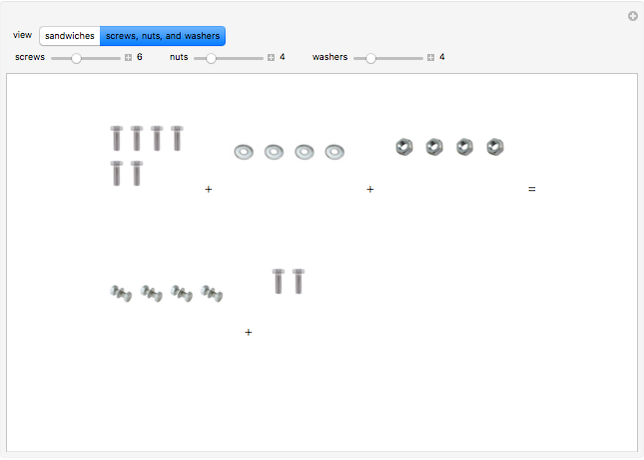
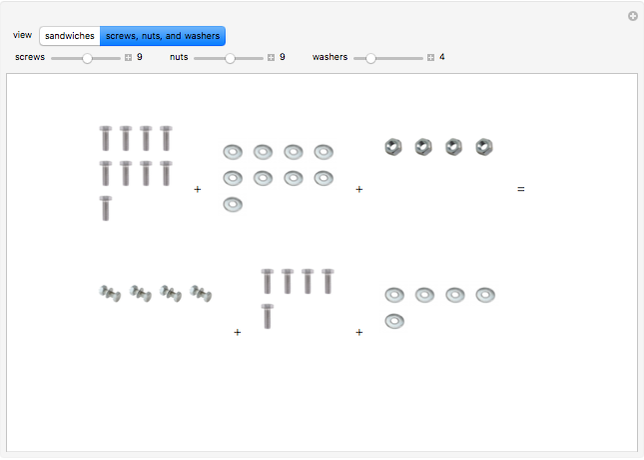
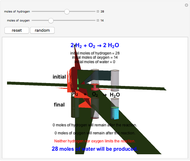
These two analogies may help you understand stoichiometry. First, imagine that you have slices of ham and bread and want to make sandwiches. You will need twice the number of slices of bread as slices of ham and that, in some cases, the numbers do not match exactly. Imagine now that the slices of bread are atoms of hydrogen and slices of ham are atoms of oxygen. Then molecules of water (with the formula 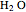 ) are formed. Just as with the sandwiches, there may be some oxygen or hydrogen atoms left over after the maximum number of water molecules is formed. The "limiting reagent" determines the amount of product that can be formed when it is completely consumed during the chemical reaction. Another example is provided using screws, washers, and nuts, which we can think of as analogous to forming hydrogen cyanide (
) are formed. Just as with the sandwiches, there may be some oxygen or hydrogen atoms left over after the maximum number of water molecules is formed. The "limiting reagent" determines the amount of product that can be formed when it is completely consumed during the chemical reaction. Another example is provided using screws, washers, and nuts, which we can think of as analogous to forming hydrogen cyanide (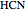 ). Each quantity can represent the amount of each element, for example 1 mol.
). Each quantity can represent the amount of each element, for example 1 mol.
Contributed by: Enrique Zeleny (February 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Analogies to Understand Stoichiometry"
http://demonstrations.wolfram.com/AnalogiesToUnderstandStoichiometry/
Wolfram Demonstrations Project
Published: February 5 2014