Chemical Potential Dependence on Temperature and Pressure

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
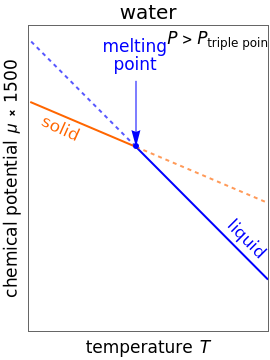
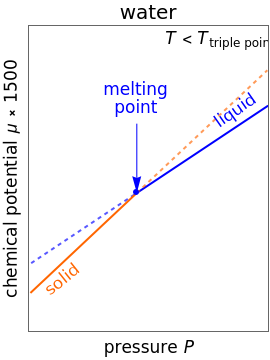
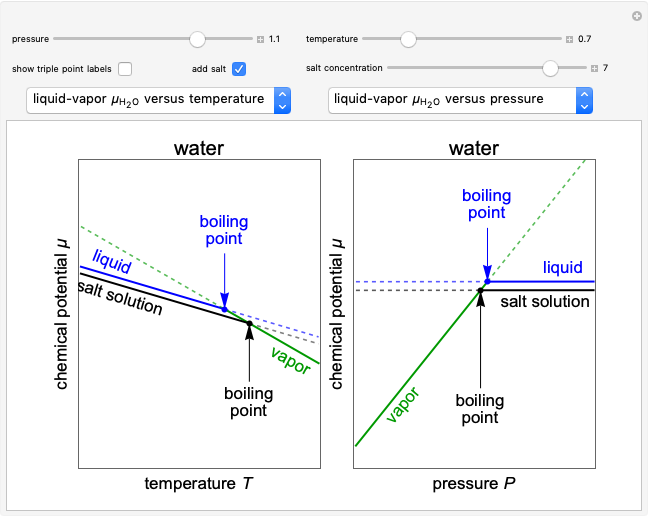
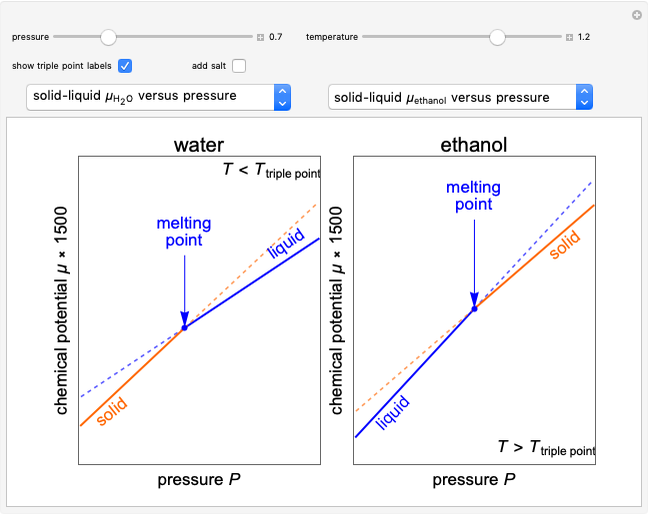
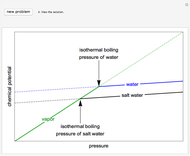
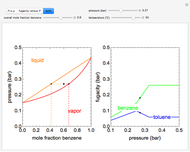
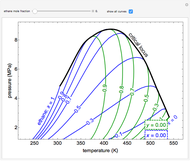
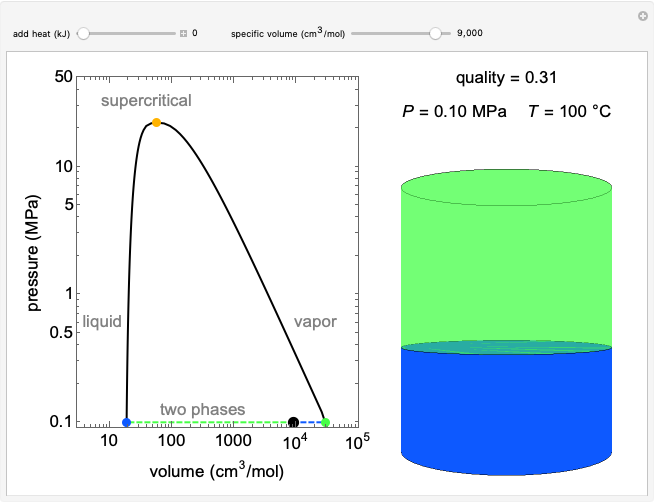
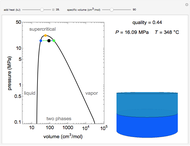
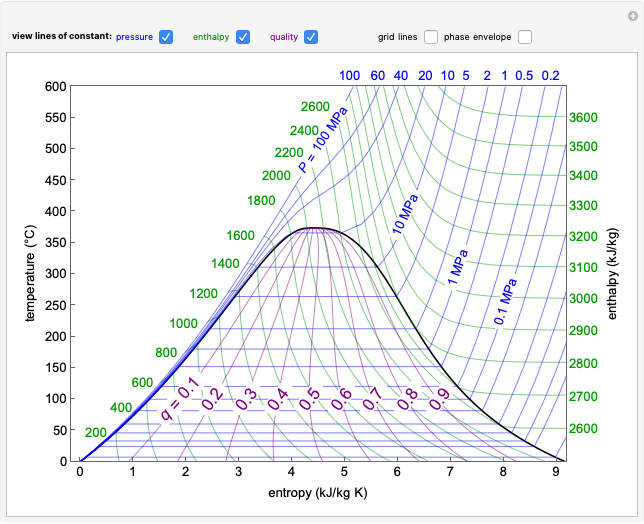
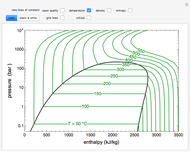
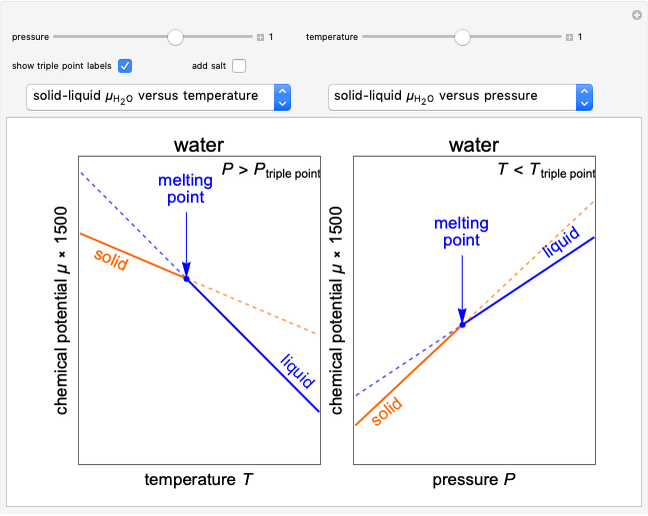
Changes in the chemical potential  of water as a function of pressure
of water as a function of pressure  (at constant temperature
(at constant temperature  ) or temperature (at constant pressure
) or temperature (at constant pressure  ) determine vapor-liquid, vapor-solid and liquid-solid phase changes. Chemical potential is given by
) determine vapor-liquid, vapor-solid and liquid-solid phase changes. Chemical potential is given by  .
.
Contributed by: Majed N. Aldossary (January 2017)
Additional contributions by: Rachael L. Baumann and John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
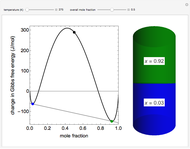
The chemical potential  is equal to the Gibbs free energy
is equal to the Gibbs free energy  for a single component. The differential of the Gibbs free energy is:
for a single component. The differential of the Gibbs free energy is:
 ,
,
where  is volume,
is volume,  is pressure,
is pressure,  is entropy and
is entropy and  is temperature. For a single component system,
is temperature. For a single component system,  .
.
Reference
[1] P. Atkins and J. de Paula, Atkins' Physical Chemistry, 8th ed., New York: Oxford University Press, 2006.
Permanent Citation