Decreasing the Freezing Point of Water with Dissolved Sodium Chloride

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
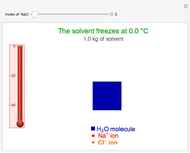
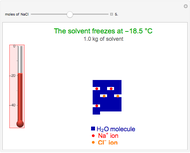
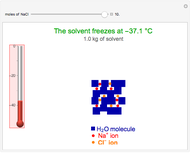
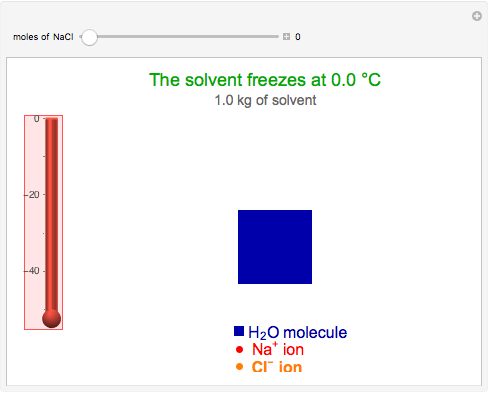
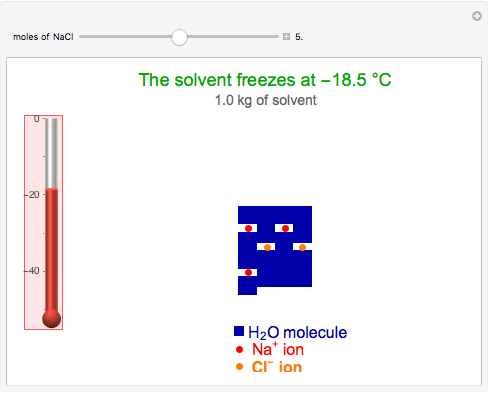
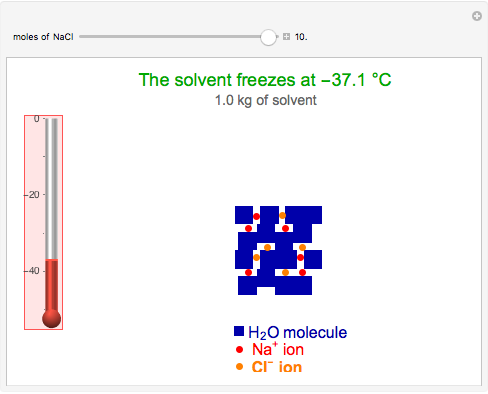
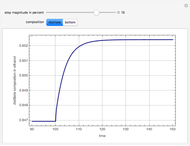
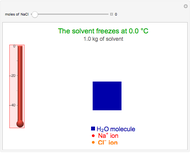
This Demonstration shows the effect of dissolved sodium chloride on the freezing point of water. Pure water, the solvent, freezes and forms a crystal lattice at zero degrees Celsius. Adding a solute, such as sodium chloride, disrupts the crystal lattice formation, resulting in a decreased freezing point. This phenomenon is represented by the equation  , where
, where  is the freezing point of the solution,
is the freezing point of the solution,  is the cryoscopic constant of the solvent,
is the cryoscopic constant of the solvent,  is the molality,
is the molality,  is the van 't Hoff factor, and
is the van 't Hoff factor, and  is the freezing temperature of the pure solvent. This linear relation is approximately valid for moderate solute concentrations. For higher concentrations, the dependence is more complex, depending on the activities of the solutions, as described, for example, by the Debye–Hückel theory.
is the freezing temperature of the pure solvent. This linear relation is approximately valid for moderate solute concentrations. For higher concentrations, the dependence is more complex, depending on the activities of the solutions, as described, for example, by the Debye–Hückel theory.
Contributed by: Percy Bromby II, Brittney Brady, Christopher Hilbrich, and Jon Knight (December 2013)
(Indiana University Northwest, Department of Chemistry, Physics, and Astronomy)
Open content licensed under CC BY-NC-SA