Equilibrium Water Activity of Binary Dry Mixtures

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
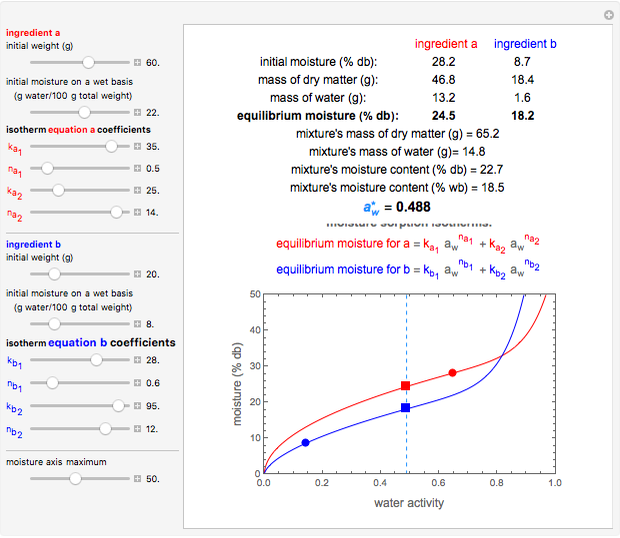
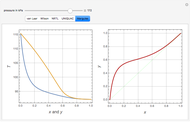
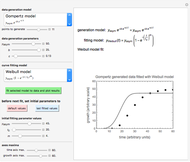
This Demonstration shows the equilibration of moisture in binary mixtures of various compositions stored together in a sealed container. It shows how moisture is lost by the wetter ingredient and gained by the drier one so that both will reach the same equilibrium water activity. It plots the moisture sorption isotherms of the two ingredients, their initial moisture contents and the water activities initially and after equilibration. It also displays the isotherm equations and other relevant quantities.
Contributed by: Mark D. Normand, Maria G. Corradini, and Micha Peleg (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
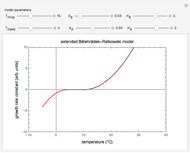
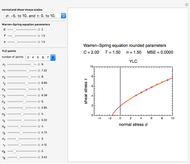
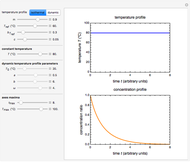
Snapshot 1: equilibration of a binary mixture of ingredients having sigmoid moisture sorption isotherms where ingredient  loses moisture and
loses moisture and  gains moisture
gains moisture
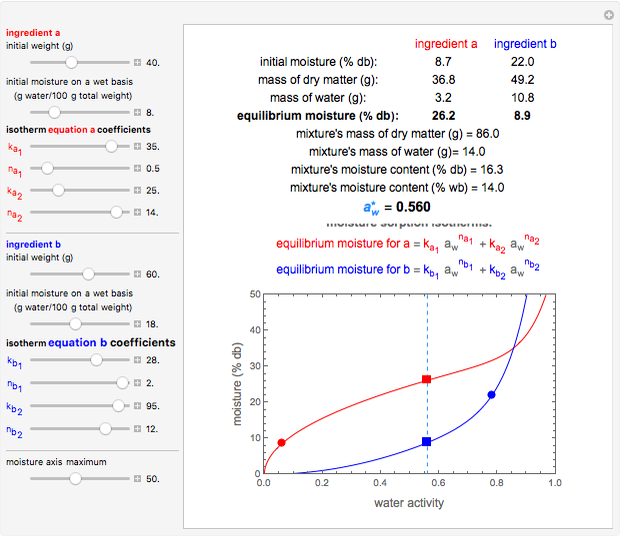
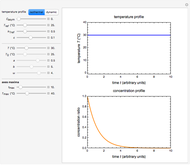
Snapshot 2: equilibration of a binary mixture of ingredients, one having a sigmoid moisture sorption isotherm and the other a non-sigmoid type I isotherm
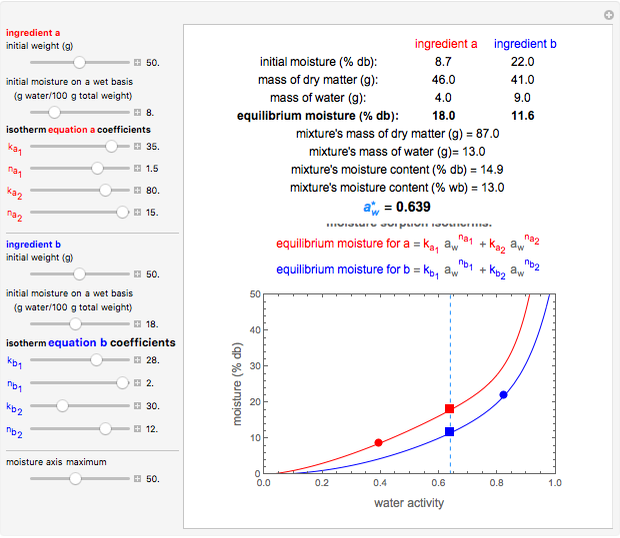
Snapshot 3: equilibration of a binary mixture of ingredients, both having a non-sigmoid moisture sorption isotherm of type I
Snapshot 4: equilibration of a binary mixture of ingredients, both having a non-sigmoid moisture sorption isotherm of type II
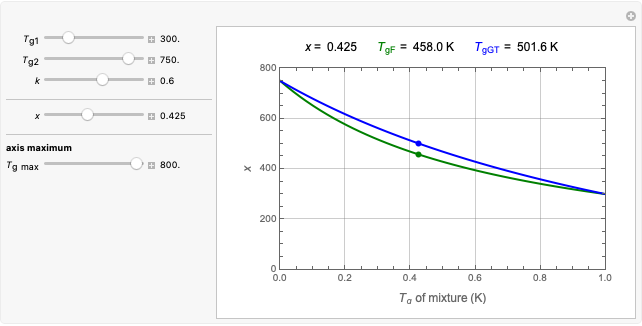
This Demonstration calculates the equilibrium water activity of a mixture of two ingredients,  and
and  , stored in a sealed container where moisture can only migrate between them. The input parameters are the components's mass (in grams), their moisture content (in percentage terms on a wet basis), and four coefficients that determine their moisture sorption isotherm equations, all set by sliders. The maximum value of the moisture axis on the plot is also entered with a slider.
, stored in a sealed container where moisture can only migrate between them. The input parameters are the components's mass (in grams), their moisture content (in percentage terms on a wet basis), and four coefficients that determine their moisture sorption isotherm equations, all set by sliders. The maximum value of the moisture axis on the plot is also entered with a slider.
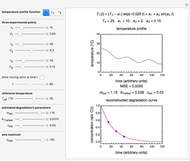
The Demonstration also calculates and displays the ingredients's dry mass, absolute mass of water, and moisture content on a dry basis before and after equilibration. It plots the two ingredients's moisture sorption isotherm curves, on which the initial and equilibrium conditions are marked as disks and squares, respectively. Details of the moisture sorption isotherm model and numerical calculation of the equilibrium water activity can be found in the first two references cited below.
Notice that not all permitted parameter combinations correspond to physical systems.
References:
M. Peleg and M. D. Normand, "Estimation of the Water Activity of Multicomponent Dry Mixtures," Trends in Food Science & Technology, 3, 1992 pp. 157–160.
M. Peleg, "Assessment of a Semi-Empirical Four Parameter General Model for Sigmoid Moisture Sorption Isotherms," Journal of Food Process Engineering, 16, 1993 pp. 21–37.
W. Wolf, W. E. L. Spiess, and G. Jung, Sorption Isotherms and Water Activity of Food Materials, New York: Elsevier, 1985.
H. A. Iglesias and J. Chirife, Handbook of Food Isotherms: Water Sorption Parameters for Food and Food Components, New York: Academic Press, 1982.
M. Peleg and M. D. Normand, Calculating Water Activity of Dry Food Mixtures.
Permanent Citation