Effect of Temperature on Chemical Equilibrium

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
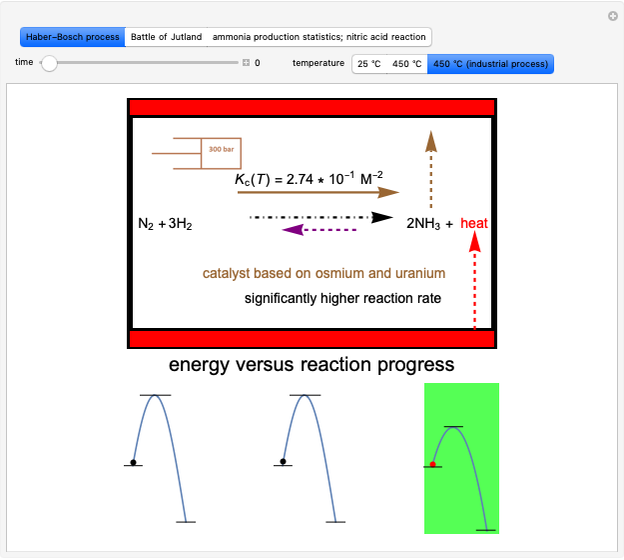
This Demonstration shows the effect of temperature on chemical equilibrium by changing temperature in an exothermic and in an endothermic reaction, specifically:
[more]
Contributed by: D. Meliga, L. Lavagnino and S. Z. Lavagnino (July 2020)
Additional contribution by: S. Porta
Open content licensed under CC BY-NC-SA
Snapshots
Details
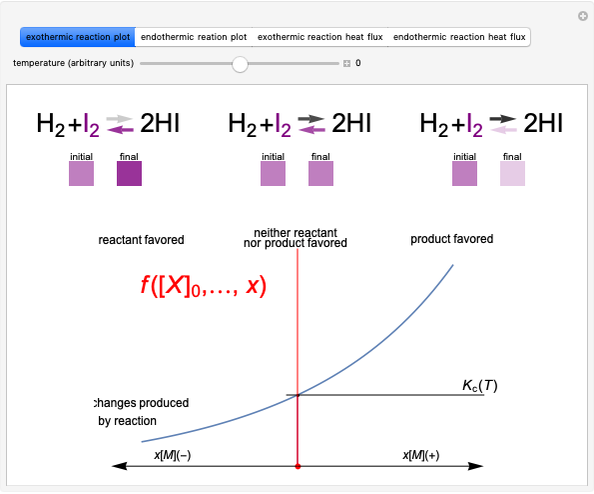
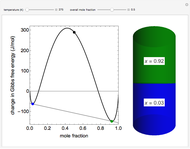
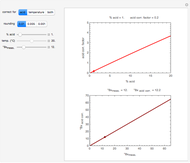
Snapshot 1: system at the equilibrium: the initial concentration fulfils the equilibrium constant equation, so there is no change
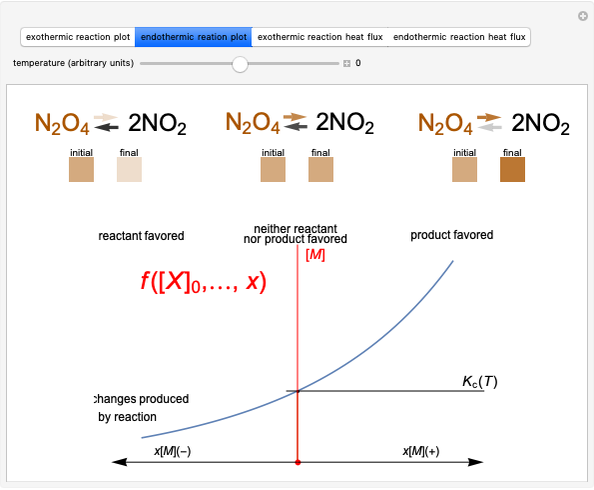
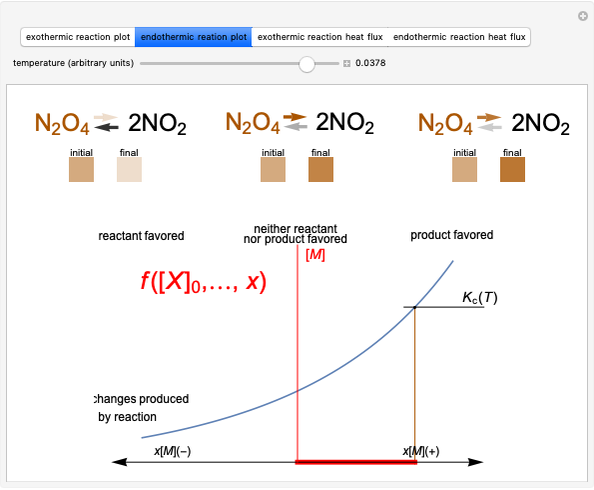
Snapshot 2: Le Chatelier's principle: raising the temperature in the equilibrium state causes a shift of an endothermic reaction toward the product side
Snapshot 3: lowering the temperature of an exothermic reaction increases the value of  and consequently shifts the equilibrium toward the product side
and consequently shifts the equilibrium toward the product side
Snapshot 4: raising the temperature of an endothermic reaction increases the value of  and consequently shifts the equilibrium toward the product side
and consequently shifts the equilibrium toward the product side
References
[1] C. H. P. Lupis, Chemical Thermodynamics of Materials, New York: North-Holland, 1983.
[2] S. V. Lavagnino. Chemical Equilibrium [Video]. (Jul 10, 2020) www.youtube.com/watch?v=TDBQOF7M-W8&list=PLswwssc6Q2yac7AM3x5UjmesLQaye-xMP&index=3.
Permanent Citation