The Haber-Bosch Process: How Chemical Technology Shaped History

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
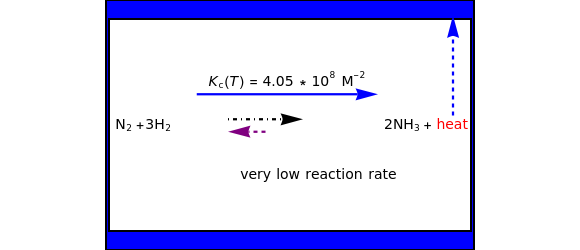
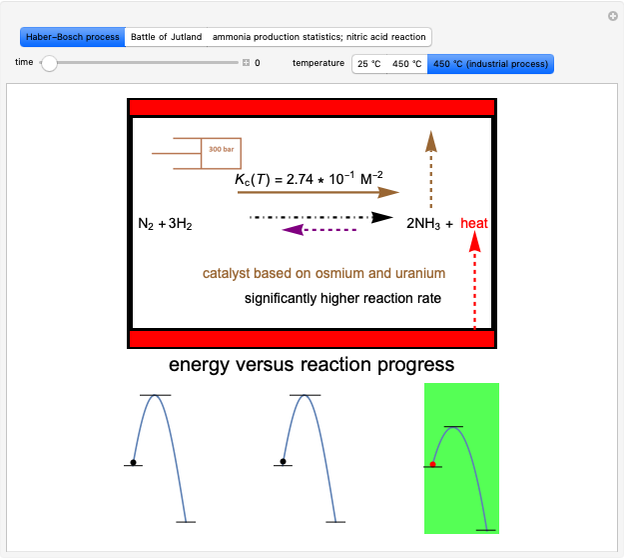
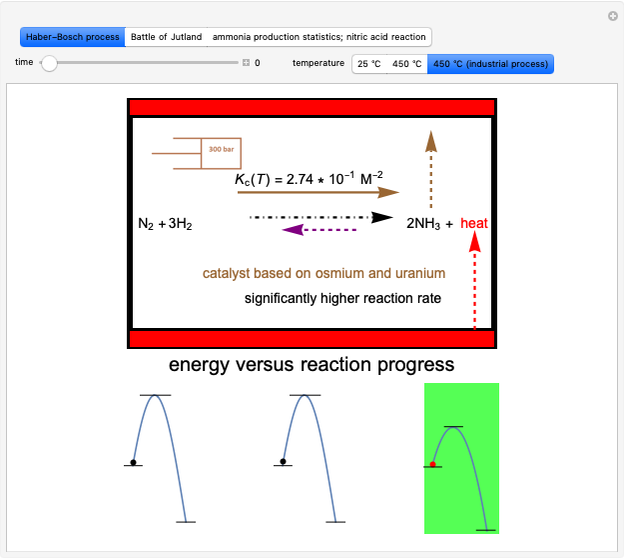
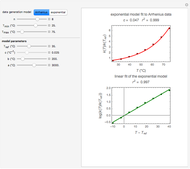
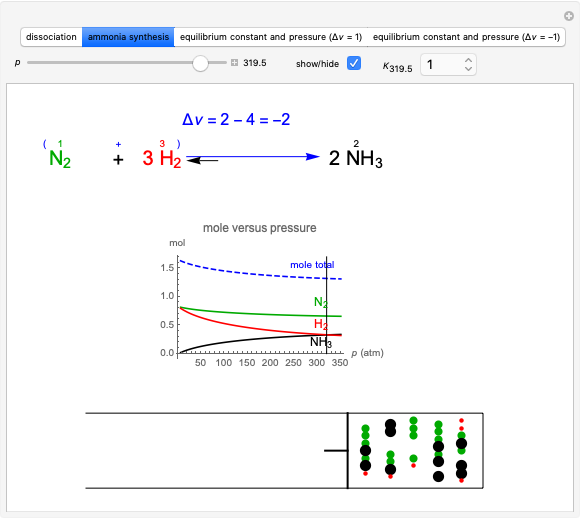
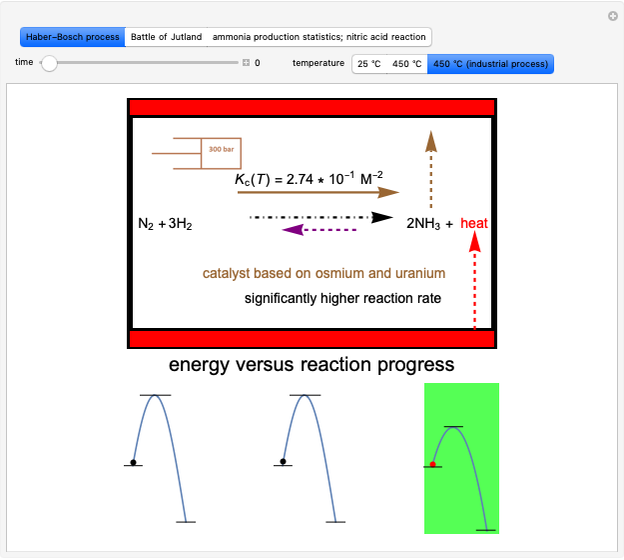
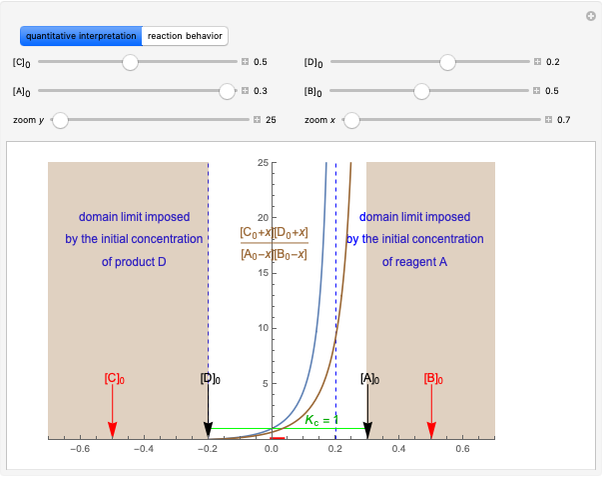
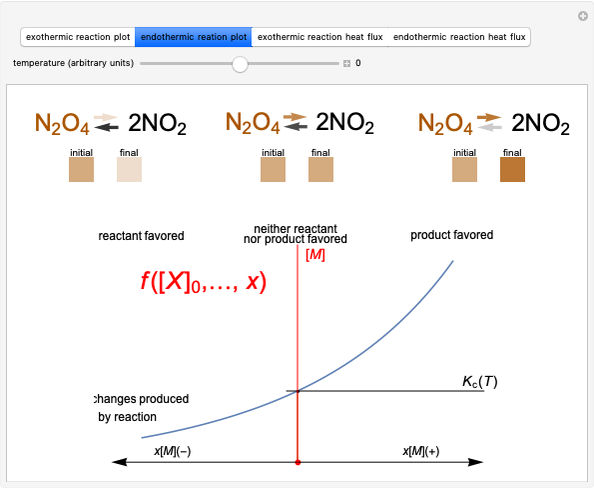
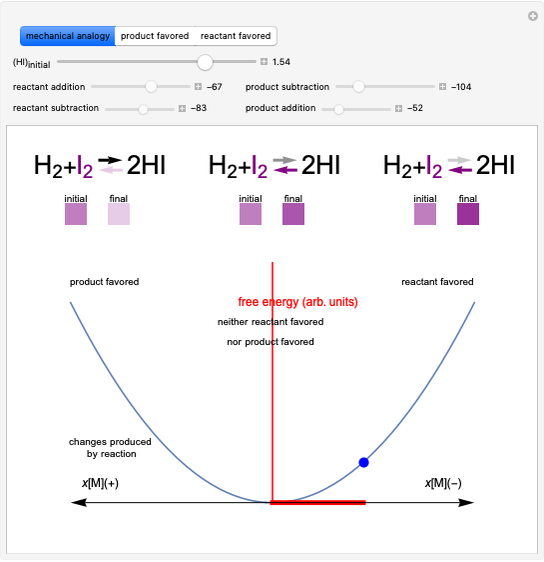
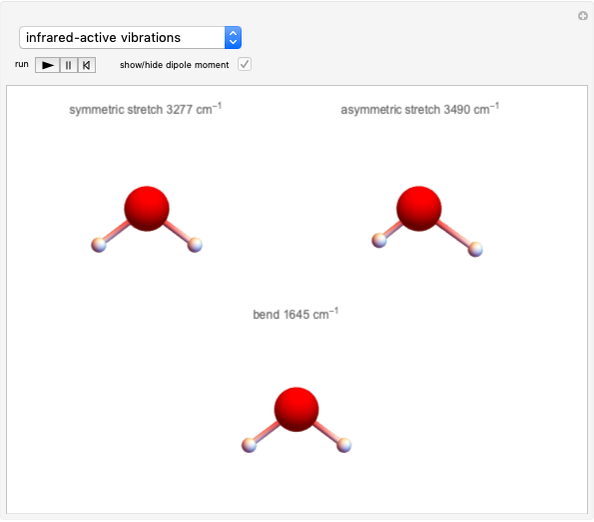
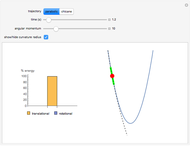
This Demonstration discusses several aspects of the industrial synthesis of ammonia. This reaction shows some unique behavior: despite being exothermic, its activation energy is remarkably high. Therefore, when the temperature is below 25 °C the thermodynamic equilibrium is shifted toward the side of the products, but the energy is too low to start the reaction. At 450 °C the reaction rate is high, but the chemical equilibrium is then shifted toward the reactant side. To make ammonia synthesis possible, it is necessary to shift the equilibrium to the right by exploiting Le Chatelier's principle. Ammonia is removed from the reaction mixture as it is formed to cause the desired shift. Finally, high pressure and an iron catalyst were used by Haber to lower the activation energy of the reaction, making the industrial process practical [1].
[more]
Contributed by: D. Meliga, L. Lavagnino and S. Z. Lavagnino (August 2020)
Additional contribution by: G. Follo
Open content licensed under CC BY-NC-SA
Snapshots
Details
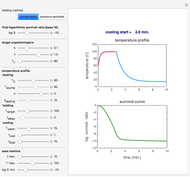
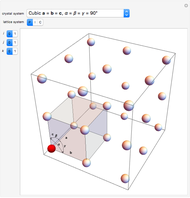
Snapshot 1: industrial process of ammonia production: high pressure, catalyst and Le Chatelier's principle enable the reaction; reactors made with special steel alloys developed by Bosch were resistant to corrosion by hydrogen
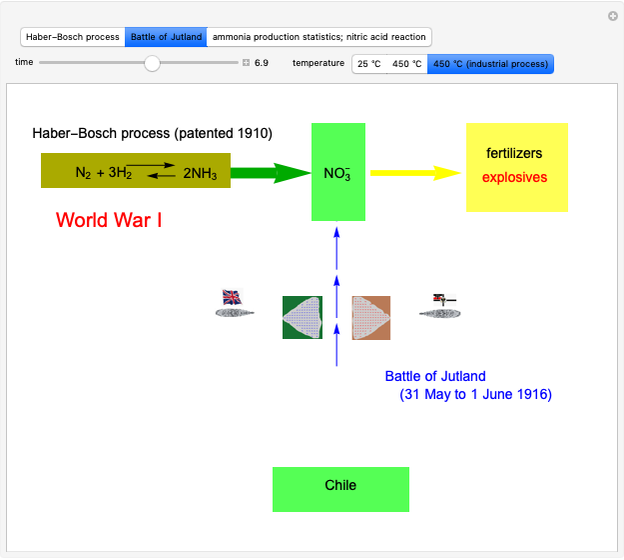
Snapshot 2: the British naval blockade leads to the Battle of Jutland; after that, the Haber–Bosch process became the main source of ammonia
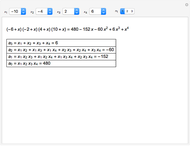
Snapshot 3: ammonia production during the years before the beginning of the war and chemical details about explosive property: reactants, products and energy released
References
[1] "La sintesi dell'ammoniaca." Scuola Zanichelli. (Aug 7, 2020). online.scuola.zanichelli.it/scopriamolachimica-files/Schede/Zanichelli_Bagatti_Scopriamo _Cap09 _S _Ammoniaca.pdf.
[2] L. Cerruti, Bella e potente, Roma: Editori Riuniti University Press, 2016.
[3] C. H. P. Lupis, Chemical Thermodynamics of Materials, New York: North-Holland, 1983.
Permanent Citation