Cryogenic Recovery of Acetone from Air

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
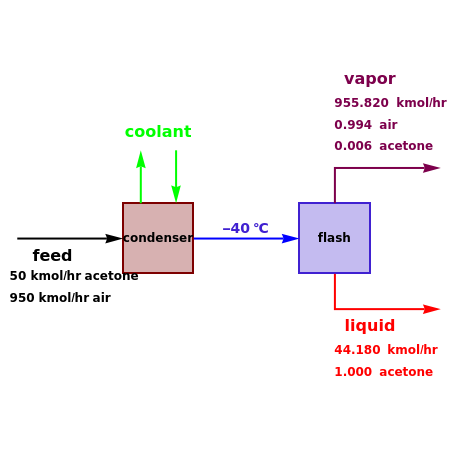
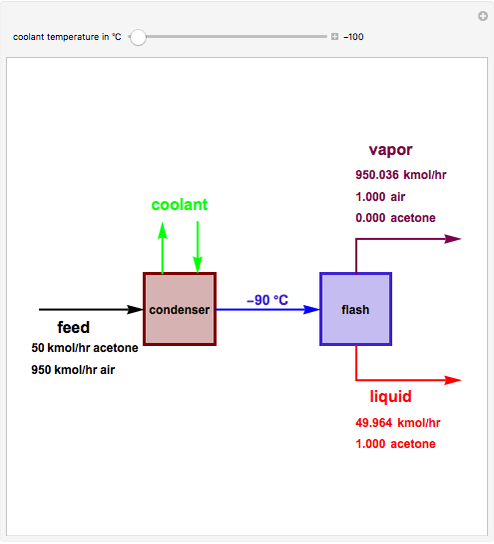
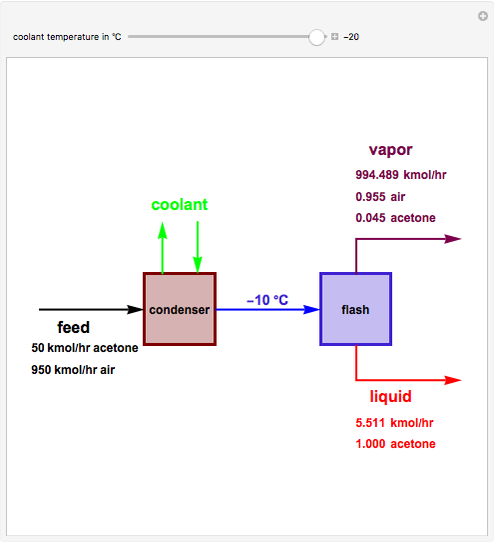
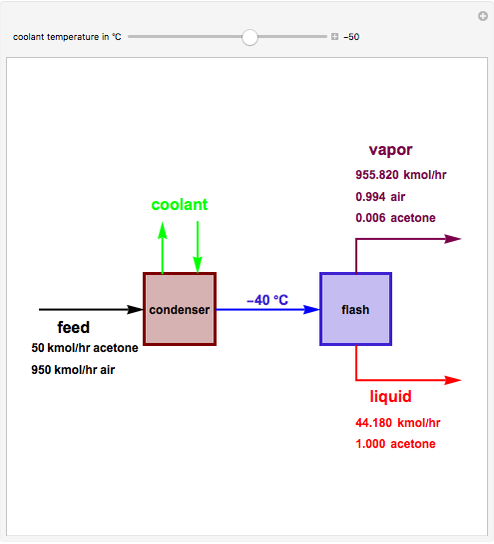
Consider a stream composed of a mixture of air and acetone with a molar flow rate of 1000 kmol/hr. The acetone content in this stream is only 5 mole%. It is still possible to recover the acetone from air using a cryogenic process, using very low temperatures  in the range
in the range  to
to  . This separation can also be called partial condensation since only acetone will condense in this temperature range. Thus, the liquid phase that exits the process will be almost pure acetone. A
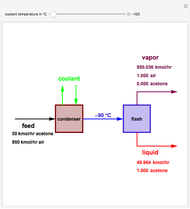
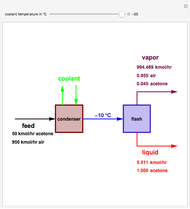
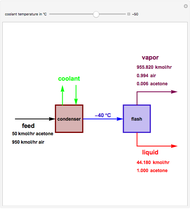
. This separation can also be called partial condensation since only acetone will condense in this temperature range. Thus, the liquid phase that exits the process will be almost pure acetone. A  approach temperature is chosen, which allows the determination of the isothermal flash temperature from the user-set value of the coolant temperature. The Demonstration displays the flow rates of the liquid and vapor stream that exits the flash drum as well as their compositions. One can see from the snapshots that the percent recovery of acetone can reach almost 100% (i.e., the liquid flow rate approaches 50 kmol/hr) when the coolant temperature is very low. This process is an alternative to countercurrent absorption of acetone in a column using water as a solvent.
approach temperature is chosen, which allows the determination of the isothermal flash temperature from the user-set value of the coolant temperature. The Demonstration displays the flow rates of the liquid and vapor stream that exits the flash drum as well as their compositions. One can see from the snapshots that the percent recovery of acetone can reach almost 100% (i.e., the liquid flow rate approaches 50 kmol/hr) when the coolant temperature is very low. This process is an alternative to countercurrent absorption of acetone in a column using water as a solvent.
Contributed by: Housam Binous and Ahmed Bellagi (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
J. M. Douglas, Conceptual Design of Chemical Processes, International ed., New York: McGraw–Hill, 1988.
Permanent Citation
"Cryogenic Recovery of Acetone from Air"
http://demonstrations.wolfram.com/CryogenicRecoveryOfAcetoneFromAir/
Wolfram Demonstrations Project
Published: March 7 2011