Stripping a Hydrocarbon from Oil Using Live Steam

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
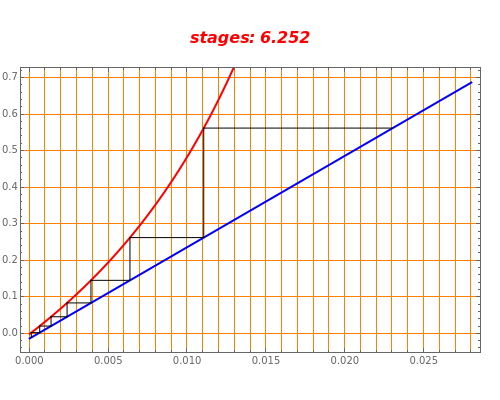
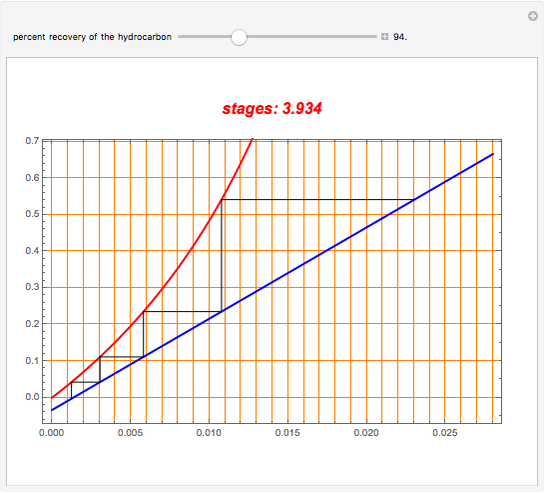
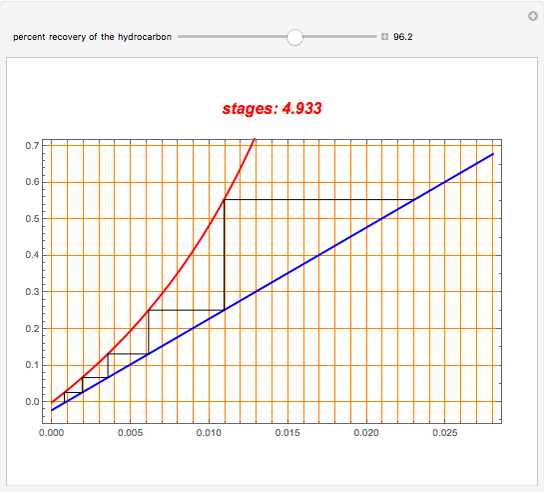
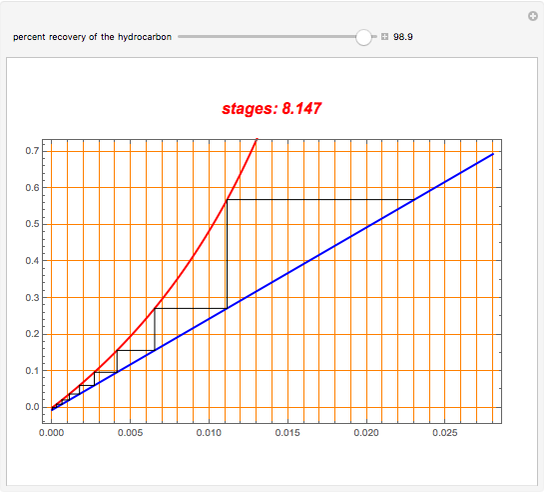
An oil containing 2.25 mole% of a hydrocarbon is stripped with live steam. Assume that 4 kmol of steam are used per 100 kmol of oil stripped. The vapor-liquid relation of the hydrocarbon in the oil is  . Assume an isothermal operation by using internal heating, so that steam does not condense in the stripping tower. This Demonstration plots the graphical construction (i.e., the operating line in blue and the equilibrium curve in red). The staircase construction, shown in black, gives the number of theoretical stages need to achieve the user-specified percent recovery of the hydrocarbon.
. Assume an isothermal operation by using internal heating, so that steam does not condense in the stripping tower. This Demonstration plots the graphical construction (i.e., the operating line in blue and the equilibrium curve in red). The staircase construction, shown in black, gives the number of theoretical stages need to achieve the user-specified percent recovery of the hydrocarbon.
Contributed by: Housam Binous (October 2009)
Open content licensed under CC BY-NC-SA
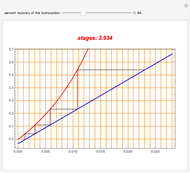
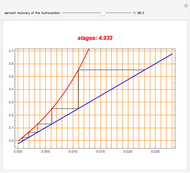
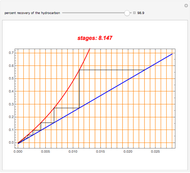
Snapshots
Details
J. M. Coulson, J. F. Richardson, J. R. Backhurst, and J. H. Harker, Coulson and Richardson's Chemical Engineering: Solutions to the Problems in Volume 2 & 3, 2nd ed., Oxford: Butterworth–Heinemann, 1997.
Permanent Citation
"Stripping a Hydrocarbon from Oil Using Live Steam"
http://demonstrations.wolfram.com/StrippingAHydrocarbonFromOilUsingLiveSteam/
Wolfram Demonstrations Project
Published: October 6 2009