Molecular Motion in Solids, Liquids, and Gases

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
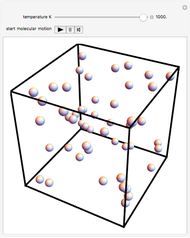
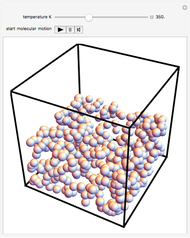
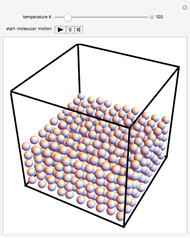
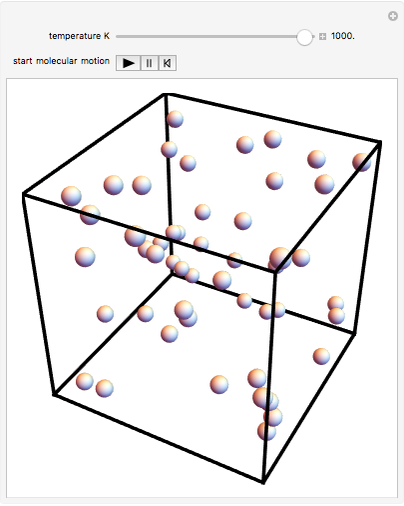
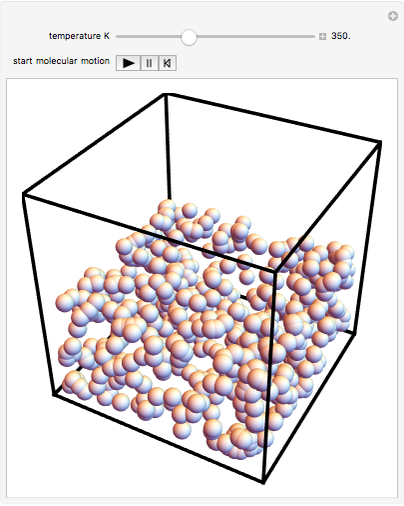
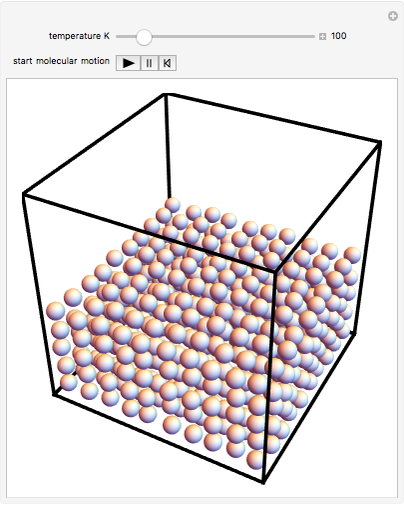
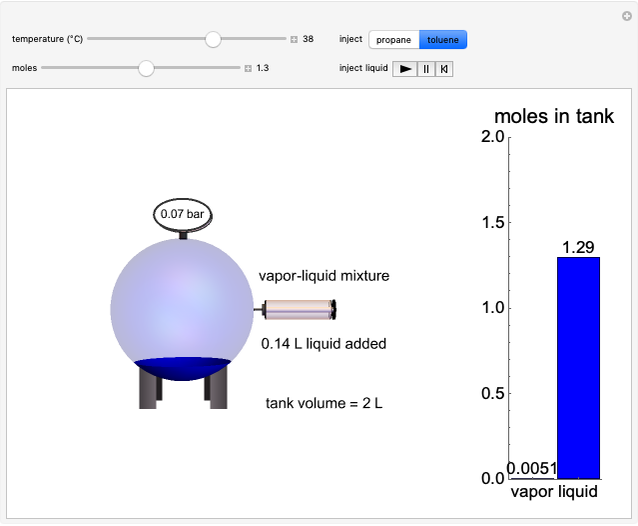
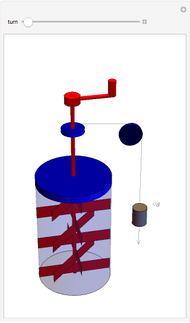
This Demonstration shows idealized representations for the molecular behavior of the three principal states of matter: solid, liquid, and gas. The hypothetical substance has a freezing point of 200 K and a boiling point of 400 K.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: gaseous state: molecules are far apart and in rapid random motion, occupying full volume of container
Snapshot 2: liquid state: molecules condense into a mass of definite volume (but variable shape) with smaller-amplitude molecular motions
Snapshot 3: solid state: molecules occupy ordered crystal lattice, vibrating about their equilibrium positions
Snapshot 4: hypothetical perfect crystal at absolute zero, when all molecular motion ceases
Permanent Citation
"Molecular Motion in Solids, Liquids, and Gases"
http://demonstrations.wolfram.com/MolecularMotionInSolidsLiquidsAndGases/
Wolfram Demonstrations Project
Published: March 7 2011