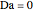Reactive Batch Rectification for Quaternary Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
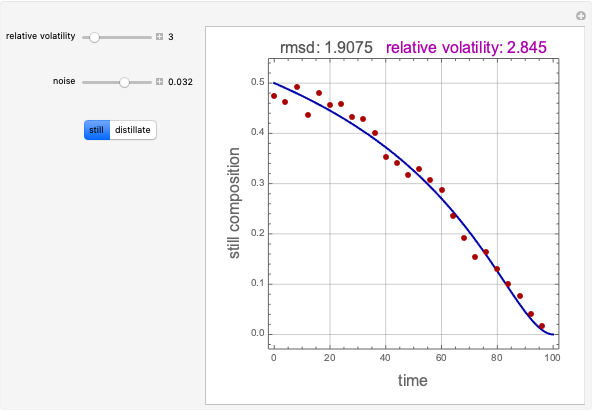
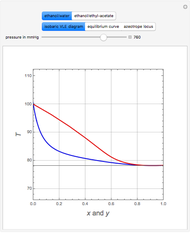
Consider an ideal quaternary mixture subject to an equilibrium-limited chemical reaction  . The relative volatilities are taken as
. The relative volatilities are taken as 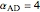 ,
, 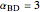 ,
, 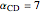 , and
, and 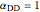 . The products
. The products  and
and 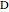 appear as the highest and lowest boiling point components of this quaternary mixture. An equimolar mixture of
appear as the highest and lowest boiling point components of this quaternary mixture. An equimolar mixture of 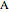 and
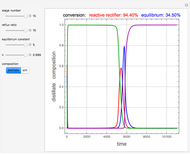
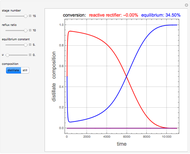
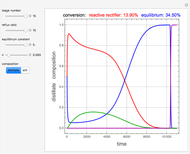
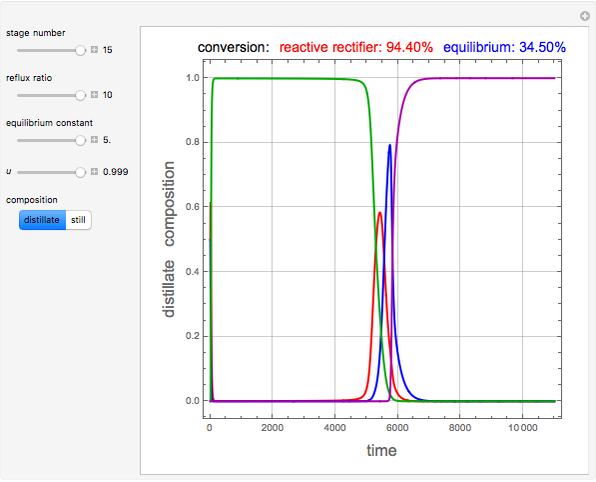
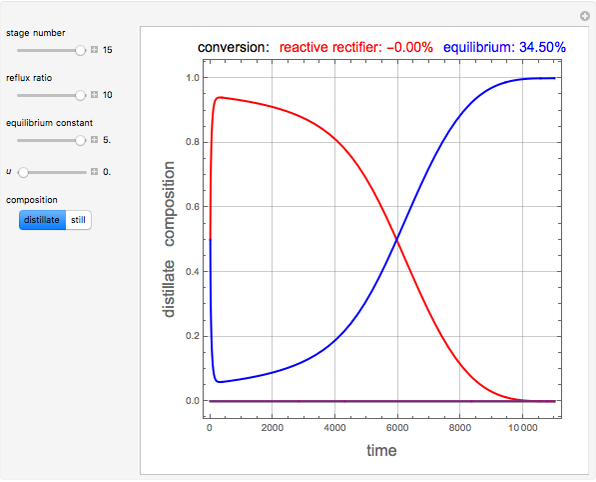
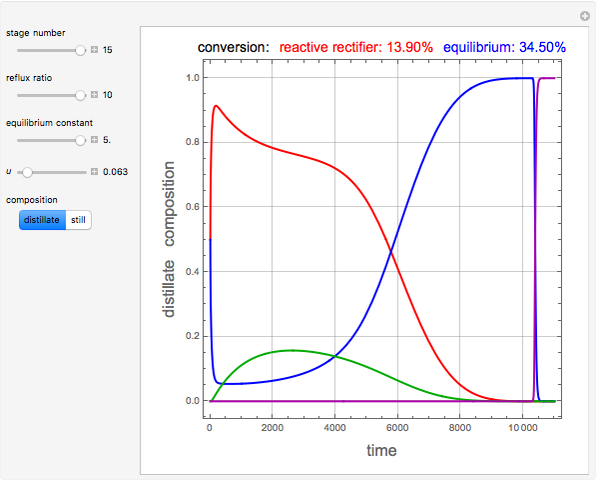
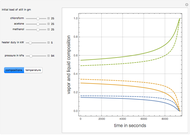
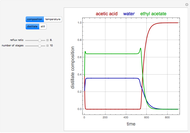
and 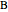 is initially fed to a reactive rectifier. For simplicity, assume that the reaction takes place only in the still. This Demonstration plots the distillate and still compositions versus time for user-set values of the Damköhler number
is initially fed to a reactive rectifier. For simplicity, assume that the reaction takes place only in the still. This Demonstration plots the distillate and still compositions versus time for user-set values of the Damköhler number 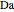 (see parameter
(see parameter 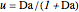 , which varies between 0 and 1, the reflux ratio
, which varies between 0 and 1, the reflux ratio 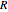 , the equilibrium constant
, the equilibrium constant 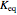 (used in the rate expression as follows:
(used in the rate expression as follows: 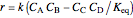 ), and the number of nonreactive stages in the rectifier
), and the number of nonreactive stages in the rectifier 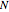 . Compositions of components
. Compositions of components 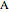 ,
, 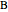 ,
,  , and
, and 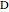 are plotted in red, blue, green, and magenta, respectively.
are plotted in red, blue, green, and magenta, respectively.
Contributed by: Housam Binous, Mamdouh Al-Harthi, and Ahmed Bellagi (December 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation