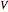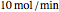Separation of Acetic Acid from Water Using Ethyl Acetate Entrainer

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
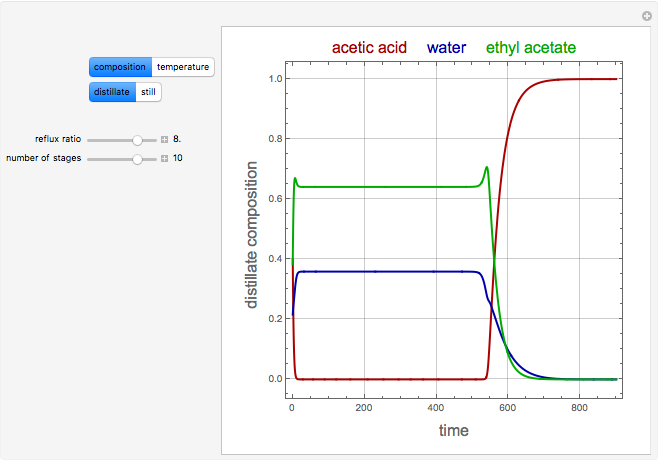
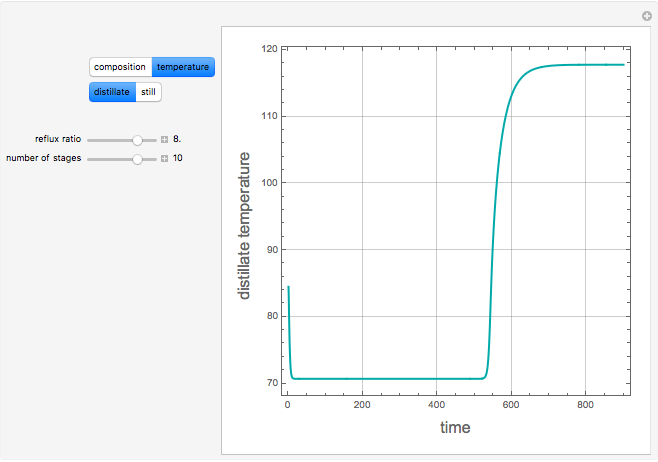
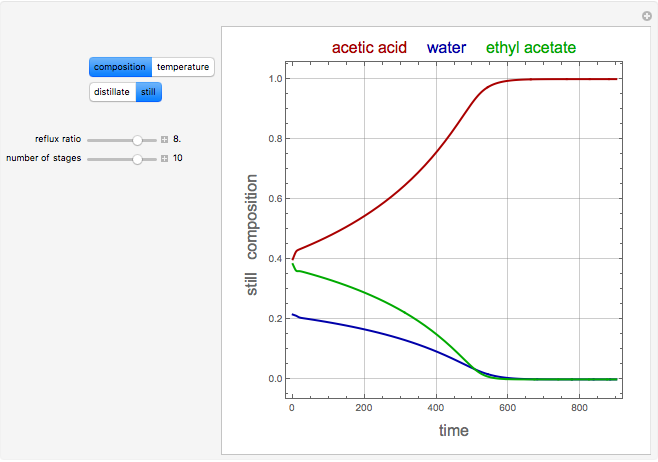
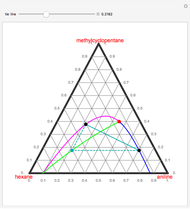
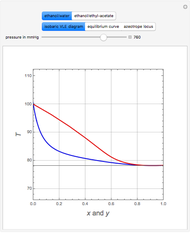
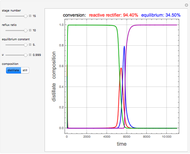
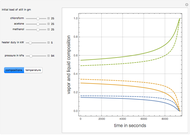
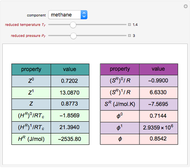
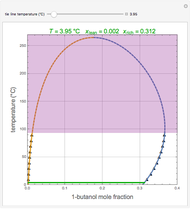
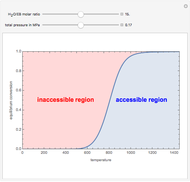
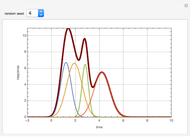
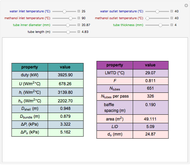
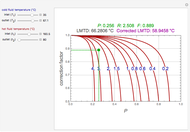
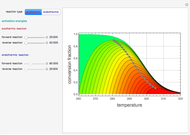
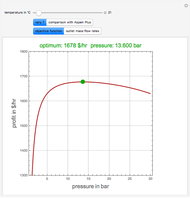
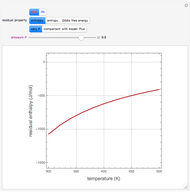
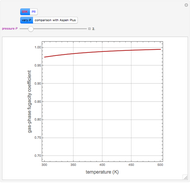
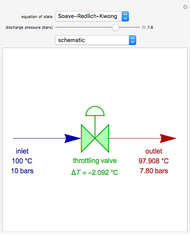
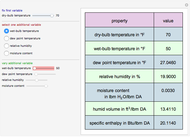
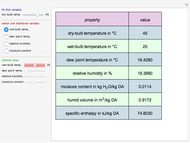
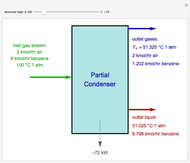
The binary mixture of acetic acid and water does not form an azeotrope. Despite this, obtaining pure acetic acid (with boiling point 118.1 °C) from a solution of acetic acid and water by distillation is very difficult because of the presence of a severe tangent pinch. Indeed, as progressive distillations produce solutions with less and less water, each further distillation becomes less effective at removing the remaining water. Distilling the solution to glacial acetic acid is therefore economically impractical. Ethyl acetate forms a positive azeotrope with water that boils at 70.4 °C (with azeotropic composition 35.9% mole water and 64.1% mole ethyl acetate). By adding ethyl acetate as an entrainer, it is possible to boil off the azeotrope and obtain pure acetic acid as the residue.
[more]
Contributed by: Housam Binous, Mamdouh Al-Harthi, and Ahmed Bellagi (December 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Separation of Acetic Acid from Water Using Ethyl Acetate Entrainer"
http://demonstrations.wolfram.com/SeparationOfAceticAcidFromWaterUsingEthylAcetateEntrainer/
Wolfram Demonstrations Project
Published: December 7 2015