Vapor-Liquid Equilibrium Using Modified Peng-Robinson Equation of State

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
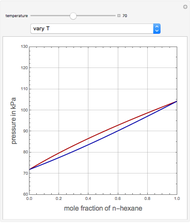
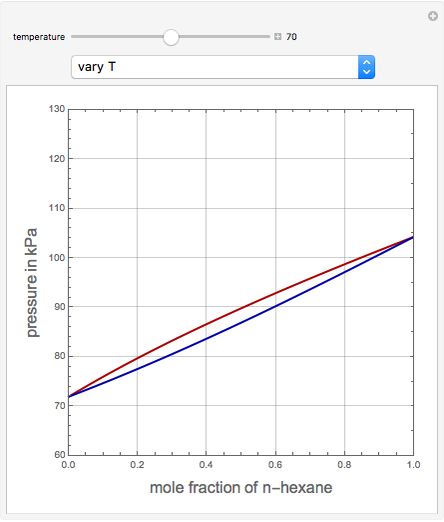
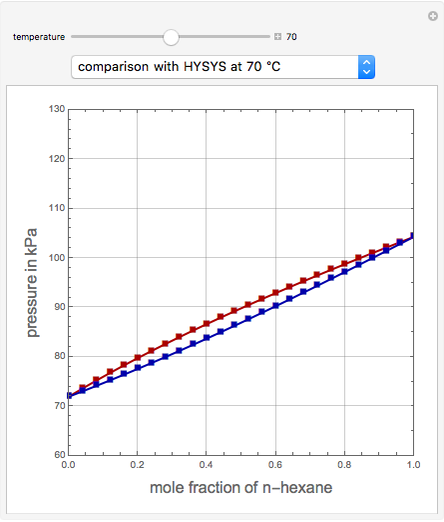
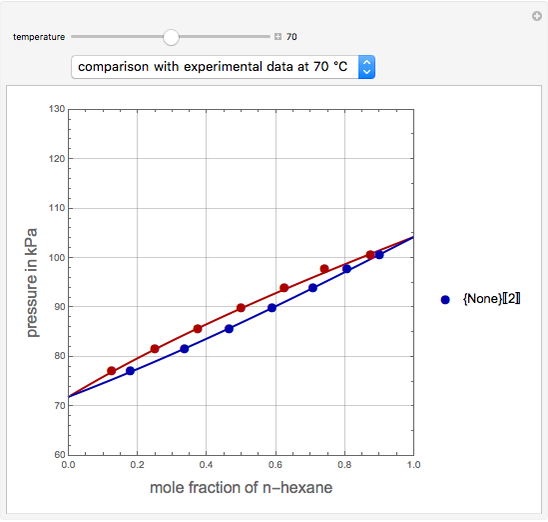
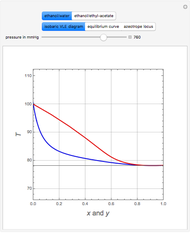
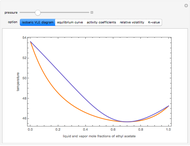
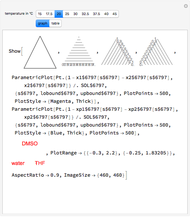
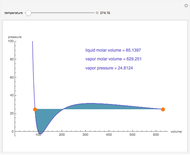
This Demonstration uses PRSV, a modification by Stryjek and Vera [1] of the Peng–Robinson equation of state (PR EoS), to give more accurate representations of isothermal vapor-liquid equilibrium (VLE) diagrams. Consider a binary mixture composed of hexane and cyclohexane. You can set the value of the temperature, 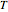 , in °C. In addition, the results at
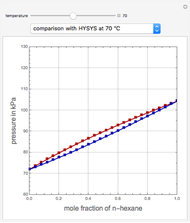
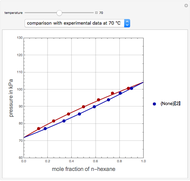
, in °C. In addition, the results at 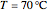 are compared to those computed by HYSYS and to the experimental values reported in [2].
are compared to those computed by HYSYS and to the experimental values reported in [2].
Contributed by: Housam Binous, Nayef M. Alsaifi, and Ahmed Bellagi (January 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
In 1986, Stryjek and Vera modified the attractive term of the Peng–Robinson equation of state (PR EoS) as follows:
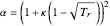 ,
,
where 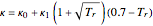 and
and 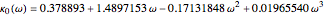 .
.
An extensive list of values of 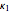 , an adjustable parameter characteristic of each pure compound, is given in Table 1 in [1].
, an adjustable parameter characteristic of each pure compound, is given in Table 1 in [1].
This new cubic equation of state, labeled PRSV, accurately predicts the vapor pressure data of many substances, including non-polar, polar non-associating, and associating compounds.
In common with the preceding cubic EoS, correlation of the vapor-liquid equilibrium data for various binary systems is possible through the binary interaction parameter 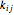 . VLE predictions, obtained with the PRSV EoS and the conventional excess Gibbs energy functions (i.e. activity coefficient models such as Wilson, NRTL, UNIQUAC, …), show close agreement except for mixtures of a polar compound (associating or not) and a saturated hydrocarbon.
. VLE predictions, obtained with the PRSV EoS and the conventional excess Gibbs energy functions (i.e. activity coefficient models such as Wilson, NRTL, UNIQUAC, …), show close agreement except for mixtures of a polar compound (associating or not) and a saturated hydrocarbon.
References
[1] R. Stryjek and J. H. Vera, "PRSV: An Improved Peng–Robinson Equation of State for Pure Compounds and Mixtures," The Canadian Journal of Chemical Engineering, 64(2), 1986 pp. 323–333. doi:10.1002/cjce.5450640224.
[2] S. T. Chen, "The Benzene-n-Hexane-Cyclo-Hexane System," Russian Journal of Physical Chemistry, 37, 1963 pp. 938–943.
Permanent Citation