Heterogeneous Kinetics by Scanning Electrochemical Microscopy

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
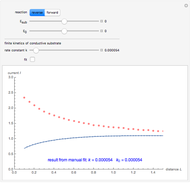
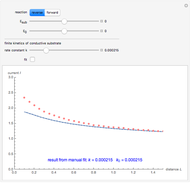
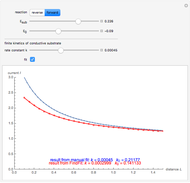
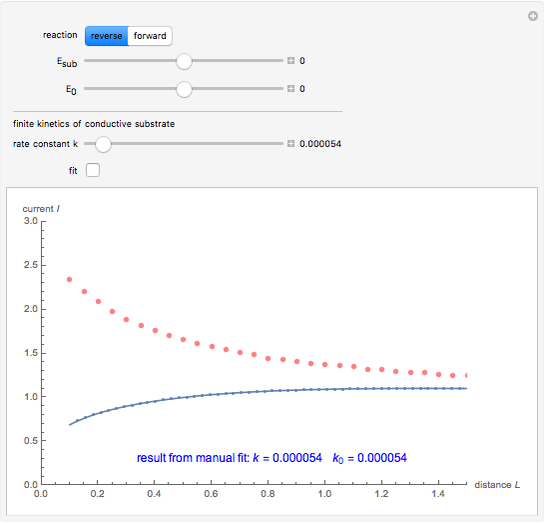
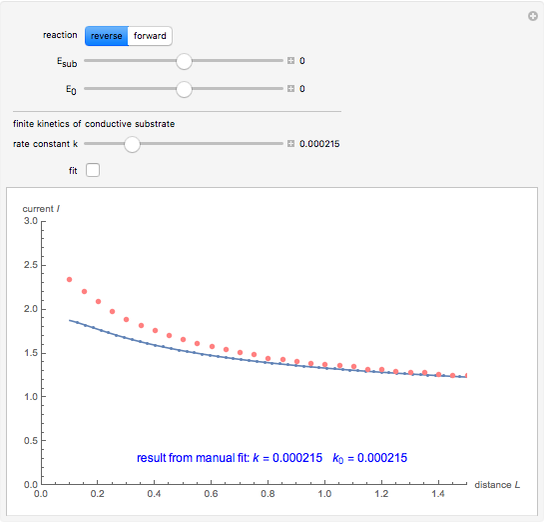
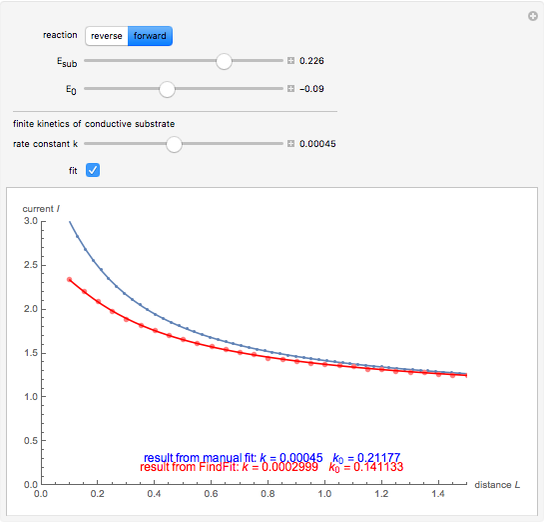
This Demonstration shows an application of Scanning Electrochemical Microscopy (SECM) to determine the heterogeneous kinetics of a conductive substrate with positive feedback mode.
Contributed by: Quang-Dao Trinh (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
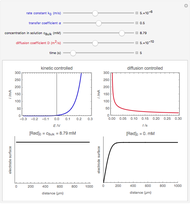
On a conductive substrate, a redox reaction  operates under kinetic control (i.e., it depends on the value of the forward rate constant
operates under kinetic control (i.e., it depends on the value of the forward rate constant  and the reverse rate constant
and the reverse rate constant  ). Hence the flux of
). Hence the flux of  feedback to the tip depends on the values of
feedback to the tip depends on the values of  and
and  .
.
In this Demonstration, you can choose the reverse ( ) or the forward reaction (
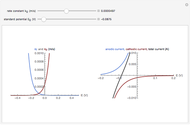
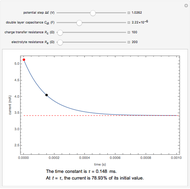
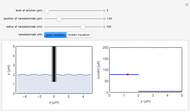
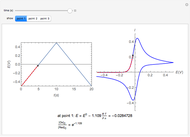
) or the forward reaction ( ) that occurs on the substrate. Then, you can manually fit the experimental data (red points) by the theoretical line (blue line) to find the reaction rate
) that occurs on the substrate. Then, you can manually fit the experimental data (red points) by the theoretical line (blue line) to find the reaction rate  . This reaction rate
. This reaction rate  can also be fitted by the Mathematica built-in function FindFit when you check the "fit" checkbox.
can also be fitted by the Mathematica built-in function FindFit when you check the "fit" checkbox.
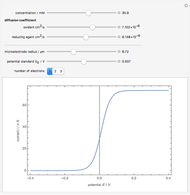
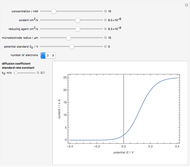
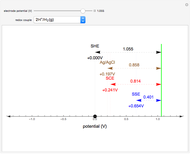
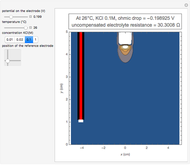
To find the standard rate constant  , you provide the potential applied to the substrate
, you provide the potential applied to the substrate  and the standard potential
and the standard potential  of the reaction on the substrate;
of the reaction on the substrate;  is found by the Butler–Volmer equation.
is found by the Butler–Volmer equation.
Reference
[1] C. G. Zoski, "Scanning Electrochemical Microscopy," Handbook of Electrochemistry, Amsterdam: Elsevier, 2007 pp. 471–540.
Permanent Citation