Quasi-Reversible and Irreversible Voltammograms at Microelectrodes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
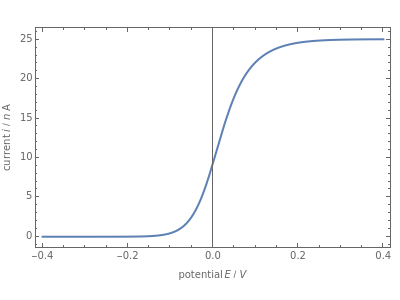
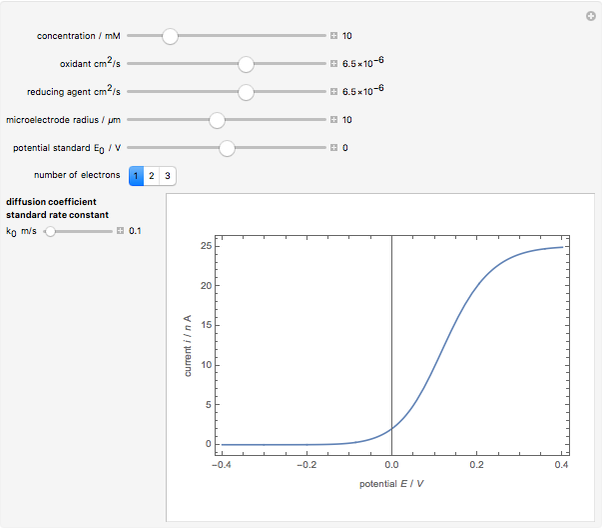
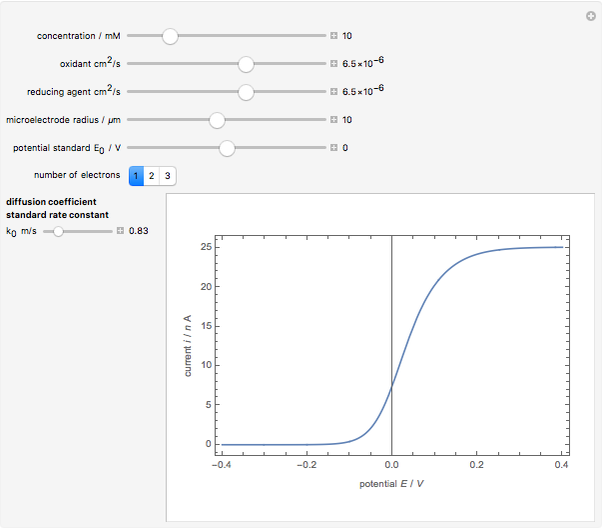
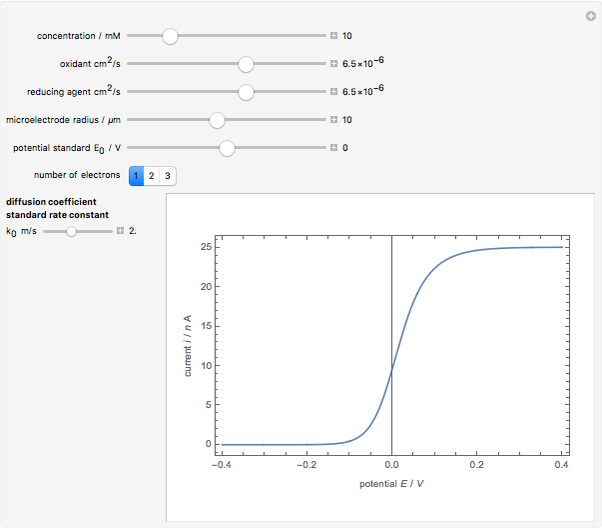
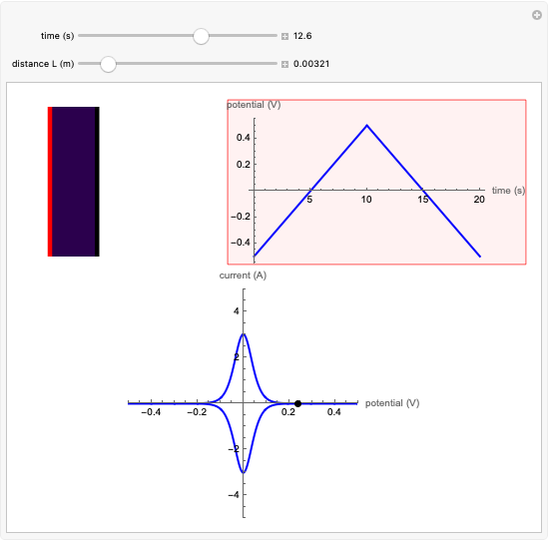
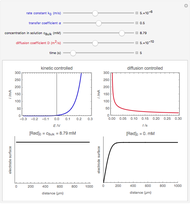
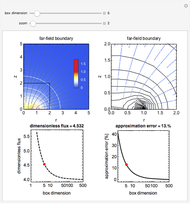
When the kinetics of the reaction occuring in cyclic voltammetry is limited, the shape of the voltammogram depends strongly on the kinetic parameter: the standard rate constant  This Demonstration shows a simulation of cyclic voltammetry with a microelectrode in steady state and under quasi-reversible and irreversible kinetics.
This Demonstration shows a simulation of cyclic voltammetry with a microelectrode in steady state and under quasi-reversible and irreversible kinetics.
Contributed by: Quang-Dao Trinh (March 2011)
Open content licensed under CC BY-NC-SA
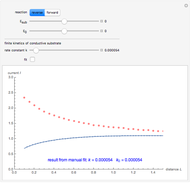
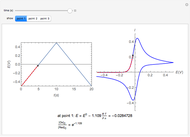
Snapshots
Details
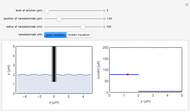
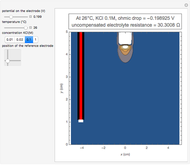
At the microelectrode, the redox reaction occurs:  . With the size of the microelectrode, the steady state is quickly attained. If the standard rate constant (
. With the size of the microelectrode, the steady state is quickly attained. If the standard rate constant (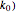 of the redox reaction on the microelectrode is limited, we find a quasi-reversible or irreversible voltammogram. One can use a dimensionless parameter
of the redox reaction on the microelectrode is limited, we find a quasi-reversible or irreversible voltammogram. One can use a dimensionless parameter 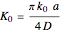 for the classification:
for the classification:
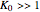 reversible
reversible
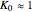 quasi-reversible
quasi-reversible
 irreversible
irreversible
According to [1], the quasi-reversible and irreversible steady-state voltammograms satisfy the equation 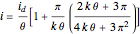 , where the limiting current of the microelectrode is given by
, where the limiting current of the microelectrode is given by 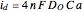 ;
;  is defined as
is defined as  , and
, and  is defined as
is defined as  .
.
 (in
(in 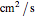 ) are the diffusion coefficients of the oxidant and reducer,
) are the diffusion coefficients of the oxidant and reducer,  (in
(in  m) is the radius of the microelectrode,
m) is the radius of the microelectrode,  (in V) is the standard potential of the redox reaction,
(in V) is the standard potential of the redox reaction,  (in V) is the applied potential,
(in V) is the applied potential,  is the gas constant (
is the gas constant (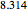 J/K mol),
J/K mol), 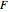 is the Faraday constant (
is the Faraday constant ( C/mol),
C/mol), 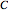 (in mol/L) is the concentration of oxidant,
(in mol/L) is the concentration of oxidant,  (in K) is the temperature,
(in K) is the temperature,  is the number of electrons transferred,
is the number of electrons transferred,  is the standard rate constant (in m/s), and
is the standard rate constant (in m/s), and  is the symmetry factor (
is the symmetry factor (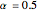 in this case).
in this case).
In this Demonstration, the kinetic parameter  controls the shape of the voltammograms; when
controls the shape of the voltammograms; when  decreases, the irreversibility increases. As
decreases, the irreversibility increases. As  tends to infinitity, we have the reversible voltammogram as shown in the Demonstration "Reversible Steady-State Voltammograms at Microelectrodes".
tends to infinitity, we have the reversible voltammogram as shown in the Demonstration "Reversible Steady-State Voltammograms at Microelectrodes".
[1] K. B. Oldham and C. G. Zoski, "Comparison of Voltammetric Steady States at Hemispherical and Disc Microelectrodes," J. Electroanal. Chem., 256(1–2), 1988 p. 11.
Permanent Citation