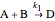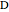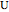Parallel Nonisothermal Reactions in Batch and Semibatch Reactors

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
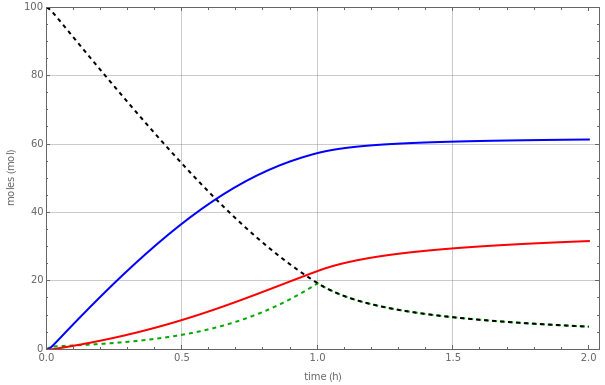
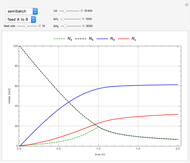
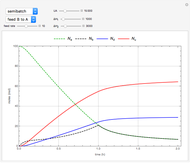
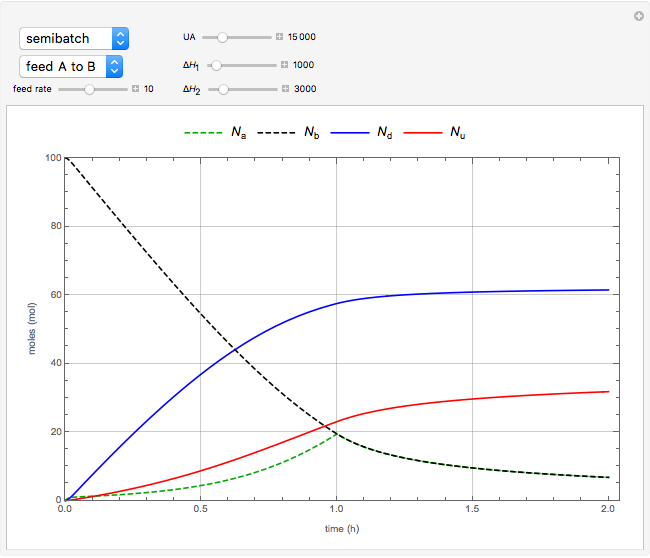
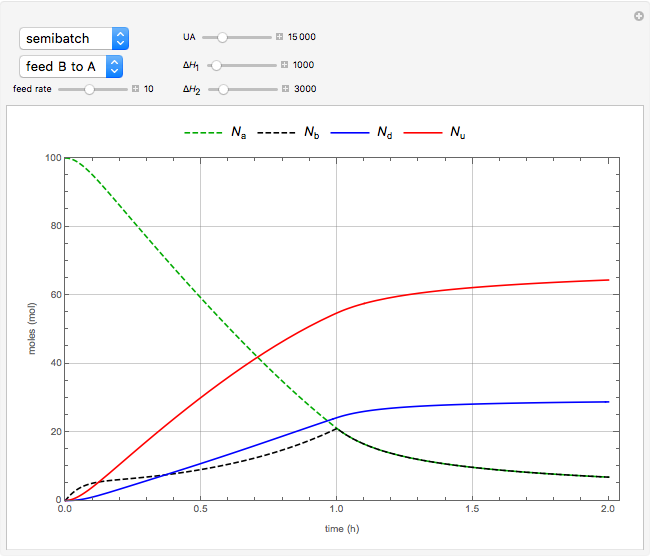
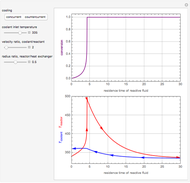
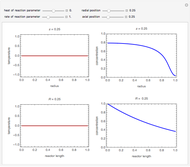
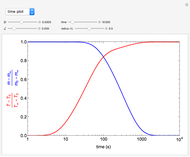
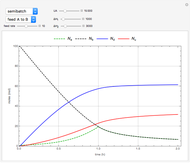
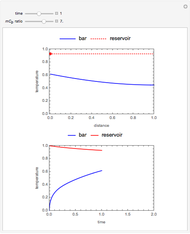
The use of semibatch reactors can be advantageous when a reaction has unwanted side reactions or a high heat of reaction. This Demonstration compares the behavior of a batch and a semibatch reactor in which a complex nonadiabatic reaction takes place.
[more]
Contributed by: Clay Gruesbeck (November 2017)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The rate laws for the reactions are:
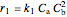 ,
,
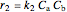 ,
,
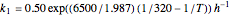 , and
, and
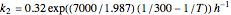
where 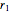 and
and 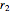 are rates of reaction,
are rates of reaction,
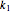 and
and 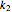 are rate constants,
are rate constants,
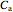 and
and 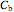 are the concentrations of
are the concentrations of 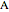 and
and 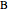 ,
,
and 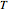 is temperature.
is temperature.
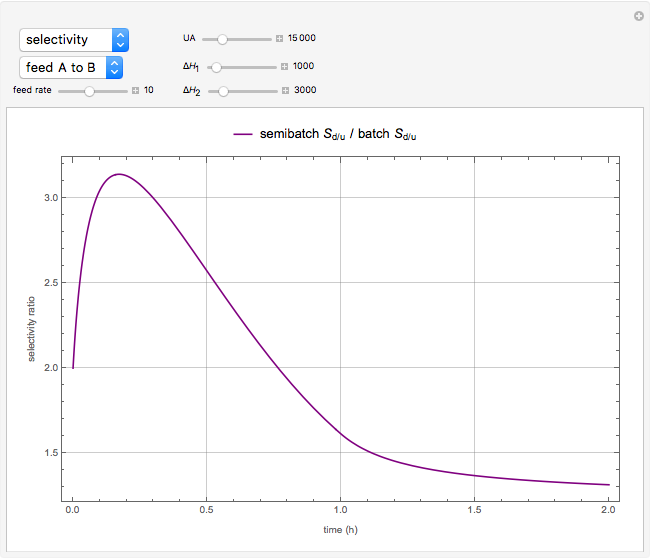
The reactant and product concentrations and selectivity in the batch reactor are:
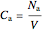 ,
,
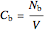 ,
,
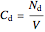 ,
,
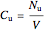 ,
,
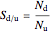 ,
,
where 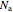 ,
, 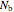 ,
, 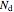 and
and 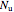 are the numbers of moles in the reactor,
are the numbers of moles in the reactor,
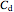 and
and 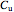 are the concentrations of the products,
are the concentrations of the products,
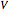 is the time-dependent volume,
is the time-dependent volume,
and 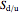 is the selectivity of the desired product.
is the selectivity of the desired product.
Material balances for the semibatch reactor when 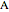 is fed into
is fed into 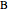 :
:
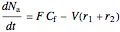 ,
,
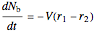 ,
,
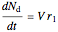 ,
,
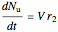 ,
,
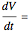
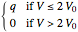 , and
, and
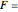
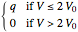 ,
,
where  is the inlet volumetric flow rate,
is the inlet volumetric flow rate,
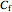 is the concentration of
is the concentration of 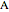 in the feed,
in the feed,
 is time,
is time,
and 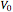 is the initial volume of the semibatch reactor.
is the initial volume of the semibatch reactor.
The material balances when 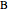 is fed into
is fed into 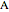 are the same except:
are the same except:
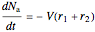 , and
, and
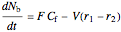 ,
,
where 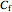 is the concentration of
is the concentration of 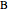 in the feed.
in the feed.
Material balances for the batch reactor are:
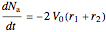 ,
,
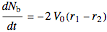 ,
,
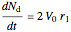 , and
, and
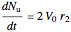 .
.
Here the concentrations are:
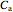 =
= 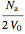 ,
,
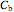 =
= 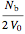 ,
,
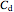 =
= 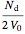 , and
, and
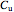 =
= 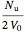 .
.
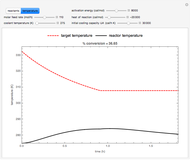
Energy balance for the semibatch reactor is:
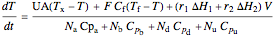 ,
,
and for the batch reactor is:
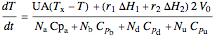
where 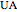 is the heat exchanger overall heat transfer coefficient,
is the heat exchanger overall heat transfer coefficient,
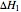 and
and 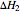 are the heat of reaction,
are the heat of reaction,
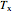 and
and 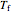 are the constant temperatures of the heat exchanger and the feed to the semibatch reactor, respectively,
are the constant temperatures of the heat exchanger and the feed to the semibatch reactor, respectively,
and 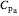 ,
, 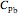 , etc. stand for heat capacities of the reactants.
, etc. stand for heat capacities of the reactants.
Reference
[1] University of Colorado Boulder. "Selectivity in a Semibatch Reactor." (Nov 8, 2017) www.colorado.edu/learncheme/kinetics/SelectivitySemibatchReactor.html.
Permanent Citation