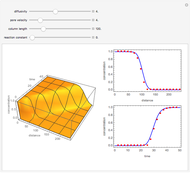
Diffusion and Reaction in a Falling Liquid Film
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
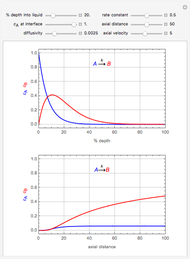
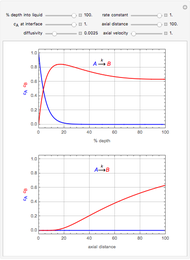
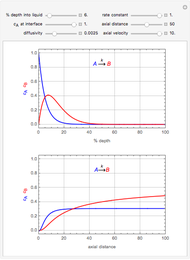
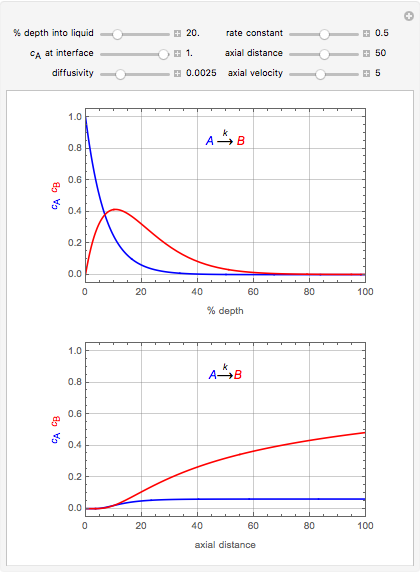
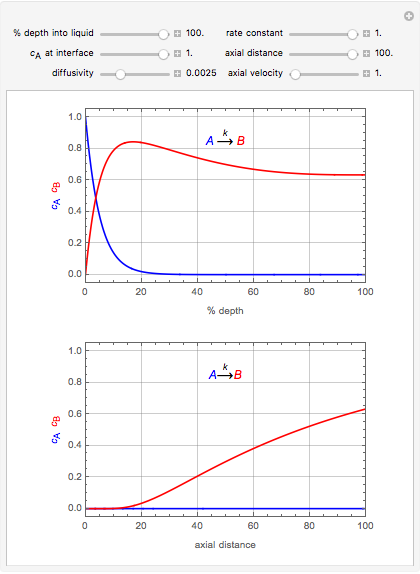
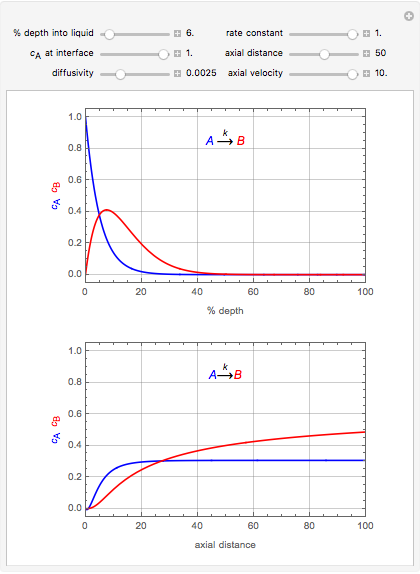
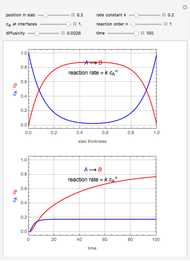
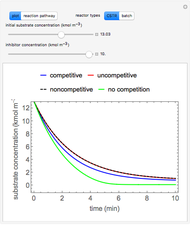
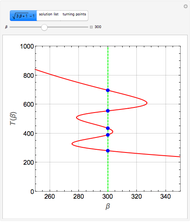
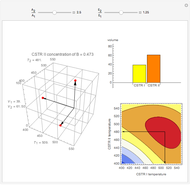
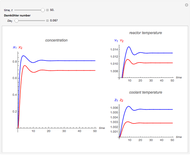
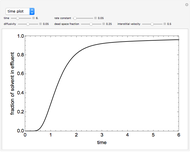
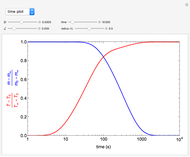
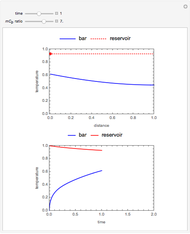
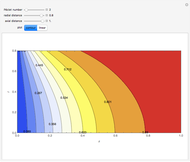
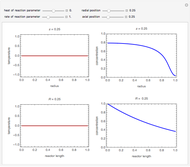
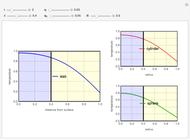
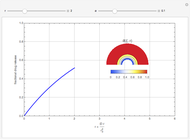
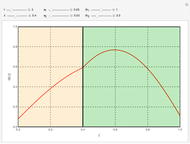
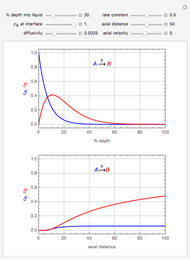
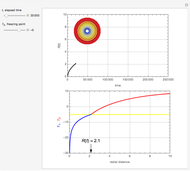
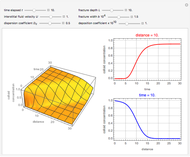
This Demonstration considers the adsorption of a gas that undergoes a first-order irreversible chemical reaction in a falling film of liquid in laminar flow.
[more]
Contributed by: Clay Gruesbeck (October 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] R. B. Bird, W. E. Stewart and E. N. Lightfoot, Transport Phenomena, rev. 2nd ed., New York: John Wiley & Sons, 2007.
Permanent Citation