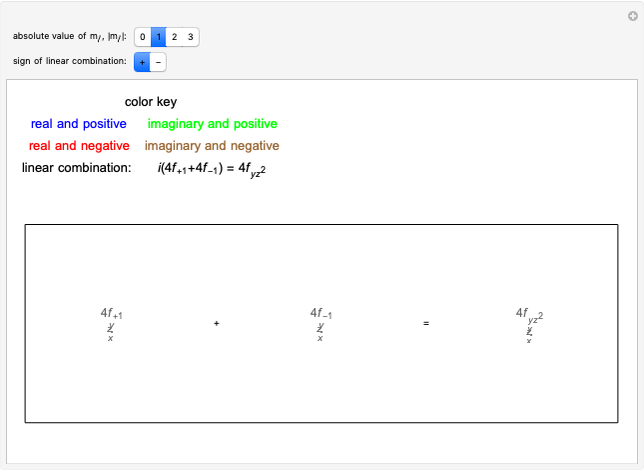
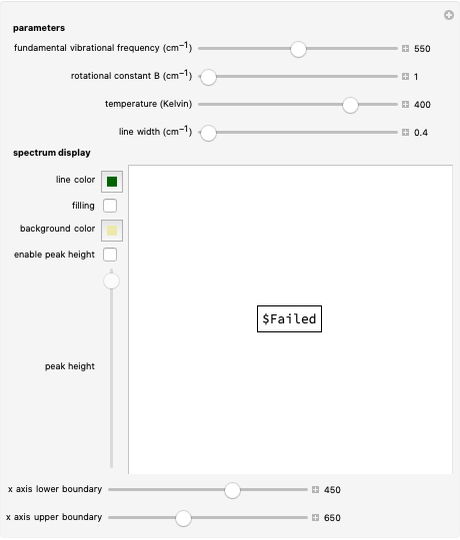
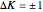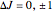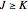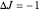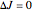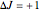Rotation-Vibration Transitions for a Perpendicular Band of a Symmetric Rotor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
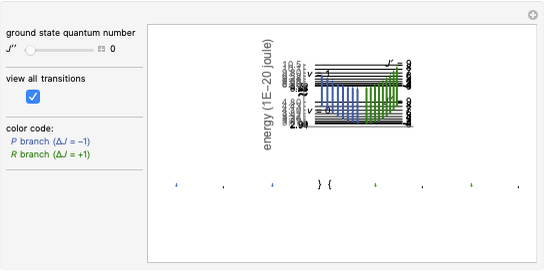
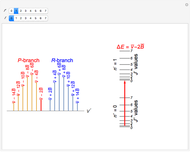
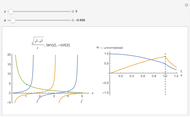
This Demonstration shows the rotation-vibration energy level transitions that correspond to the lines observed in a rotationally resolved infrared spectrum of a perpendicular band of a symmetric rotor. The inherent degeneracy of the vibrational levels (since a symmetric rotor possesses a greater-than-twofold rotation axis) has been neglected in order to maintain clarity in this Demonstration. The transitions occur between nondegenerate vibrational energy levels, and the fundamental vibrational transition is accompanied by rotational transitions in which 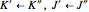 .
.
Contributed by: Whitney R. Hess and Lisa M. Goss (August 2011)
(Idaho State University)
Open content licensed under CC BY-NC-SA
Snapshots
Details
In this Demonstration, a superscript double prime (″) indicates lower state constants and quantum numbers and a superscript prime (′) indicates excited state constants and quantum numbers.
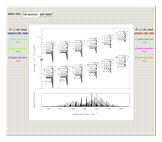
The spectrum in the bottom graphic is simulated at a temperature of 50 Kelvin and with the assumptions that the centrifugal distortion constants and anharmonicity are negligible. Unequal values for the lower and excited state rotational constants 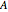 and
and 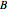 were used in order to account for the interaction of rotation and vibration
were used in order to account for the interaction of rotation and vibration 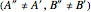 . The values of
. The values of 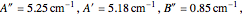 and
and 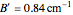 were used to simulate the spectrum.
were used to simulate the spectrum.
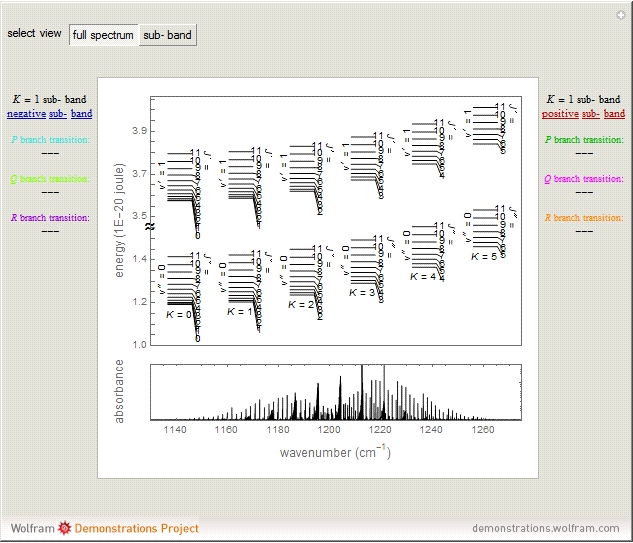
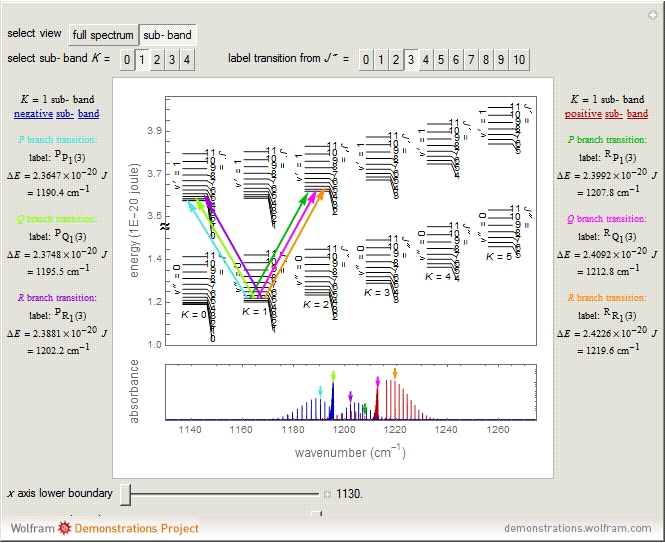
The  axis in the top graphic is arbitrary. The arrows indicating transitions and the energy levels corresponding to each value of
axis in the top graphic is arbitrary. The arrows indicating transitions and the energy levels corresponding to each value of 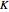 are spread out along the
are spread out along the  axis only for clarity.
axis only for clarity.
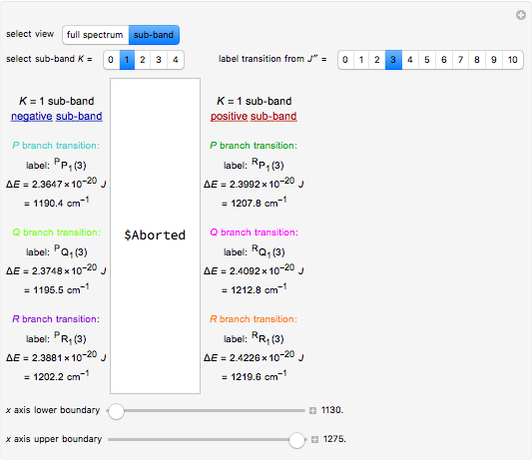
The transition labels are written as 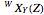 , where
, where 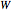 ,
, 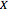 ,
, 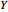 , and
, and 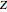 are as follows:
are as follows:
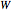 : transitions with
: transitions with 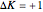 ("positive" sub-bands) are designated with a superscript
("positive" sub-bands) are designated with a superscript 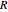 and transitions with
and transitions with 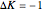 ("negative" sub-bands) are designated with a superscript
("negative" sub-bands) are designated with a superscript 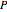 ;
;
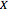 :
: 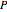 ,
, 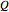 , or
, or 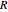 depending on whether
depending on whether 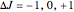 , respectively;
, respectively;
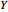 : the value of
: the value of 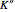 is indicated by the subscript;
is indicated by the subscript;
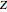 : the value of
: the value of 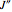 is enclosed in the parentheses.
is enclosed in the parentheses.
For example, 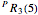 indicates the line corresponding to the
indicates the line corresponding to the 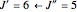 transition in the
transition in the 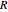 branch within the
branch within the 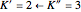 "negative" sub-band.
"negative" sub-band.
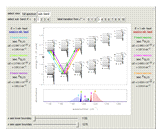
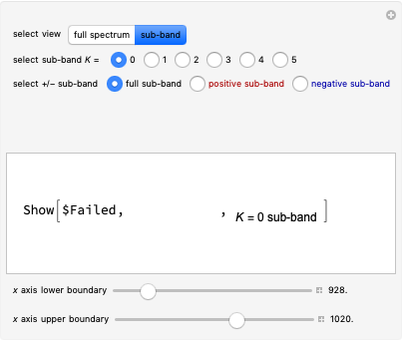
Snapshots 1 and 2: full spectrum (does not utilize the Manipulate functionality) and sub-band views, respectively
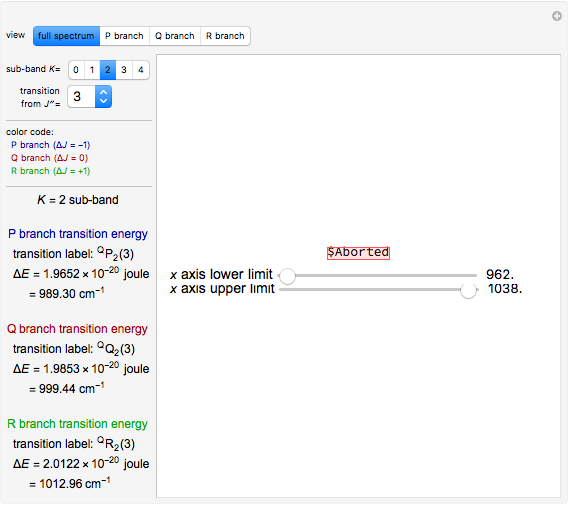
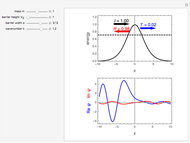
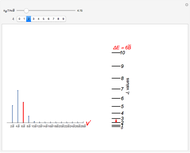
Snapshot 3: using the  axis lower and upper boundary controls will zoom in on a region of the spectrum and if a transition of interest is outside of the chosen range, the message "transition out of
axis lower and upper boundary controls will zoom in on a region of the spectrum and if a transition of interest is outside of the chosen range, the message "transition out of  axis range" will appear
axis range" will appear
Snapshot 4: the separation between the "positive" and "negative" sub-bands becomes larger as a result of the greater difference in the transition energies between the sub-bands as 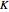 increases
increases
References
[1] P. Atkins and J. de Paula, Physical Chemistry, New York: Oxford University Press, 2006.
[2] G. Herzberg, Molecular Spectra and Molecular Structure II. Infrared and Raman Spectra of Polyatomic Molecules, Princeton, NJ: D. Van Nostrand Company, Inc., 1945.
Permanent Citation