Topologically Interesting Molecules

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
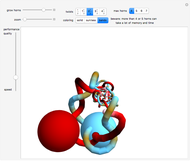
DNA and proteins show unique stereochemistry with a variety of biochemical functions. Quite interesting structures can be formed, among them catenanes and knots.
[more]
Contributed by: Guenther Gsaller (April 2013)
(Institute of Organic Chemistry, Johannes Kepler University, Linz, Austria, http://www.jku.at/orc/)
Open content licensed under CC BY-NC-SA
Snapshots
Details
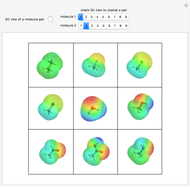
Types of Architectures [1–3, 12]
1. A catenane is a mechanically interlocked assembly with two or more macrocycles.
2. Rotaxanes contain a linear component (shaped like a dumbbell) threaded through a macrocycle.
3. Knotanes are topological isomers of macrocycles that are analogous to macroscopic knots.
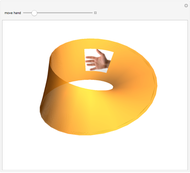
4. A Möbius strip is a ribbon closed with a twist. Since 2003, several aromatic molecules with 
 -electrons and Möbius topology have been made.
-electrons and Möbius topology have been made.
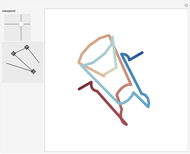
5. Borromean rings contain three intertwined macrocycles. If you remove any of the three macrocyles, the other two are not linked.
Some Comments about the Five Architectures Shown
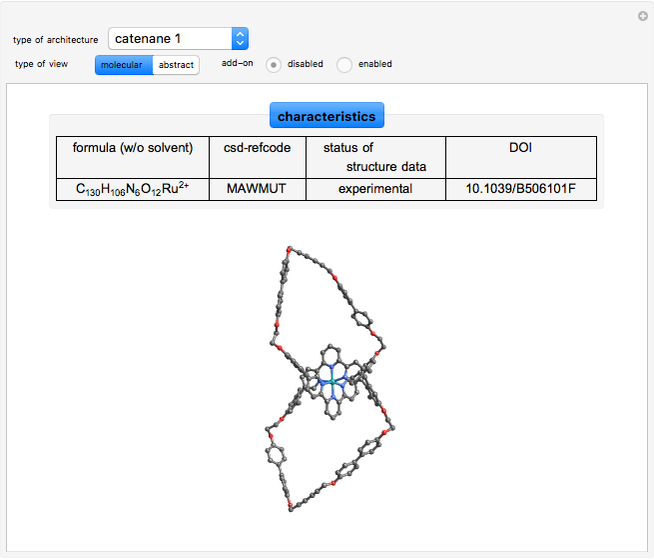
Catenanes
The Ru(terpy-biphencatenane) and the organogold(I)catenane  can be visualized topologically with two interlocked rings [4, 5].
can be visualized topologically with two interlocked rings [4, 5].
Rotaxanes
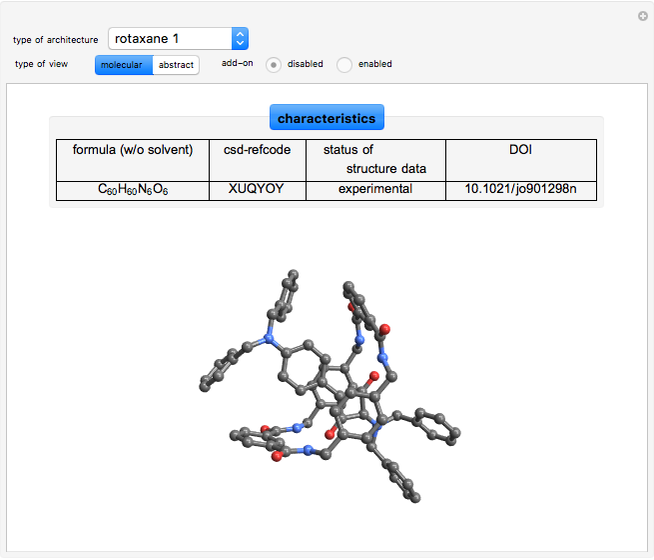
In the truncated squaraine rotaxane the ring structure looks like a boat. A [3]pseudorotaxane was formed by dialkyl-viologens and dibenzo-24-crown-8. It contains two macrocycles. Rotaxanes can be visualized topologically with a stick formed like a dumbbell threaded through one or more rings [6, 7].
Knotanes
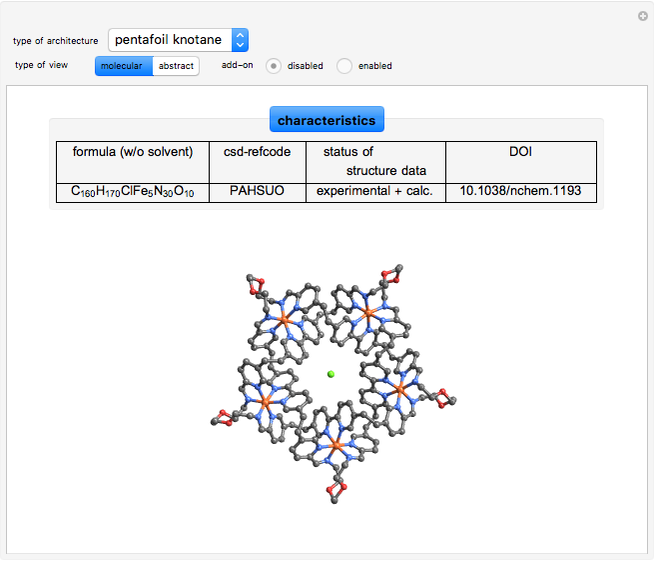
The organic ligand 7-((13-(quinolin-6-yloxy)-3,5,8,11-tetraoxatridecyl)oxy)quinoline, named 5 in [8], can form a trefoil knotane  . An assembly of five bis-aldehyde and five bis-amine building blocks around a chloride anion was used to synthesize a pentafoil knotane [9]. These compounds can be visualized topologically with the trefoil and the Solomon's seal knot.
. An assembly of five bis-aldehyde and five bis-amine building blocks around a chloride anion was used to synthesize a pentafoil knotane [9]. These compounds can be visualized topologically with the trefoil and the Solomon's seal knot.
Möbius Strips
Both hexapyrins contain a structure with Möbius topology. To visualize it approximately, enable the add-on. The red part of the strip is a signal for the twist. The abstract view of the Möbius strip delivers a one-sided surface [10, 11].
Borromean Rings
The chiral borromeate contains three rings. To visualize it, enable the add-on. Borromean rings are the simplest example for a Brunnian link [13].
Status of Structure Data
Options for the structure data status are:
experimental, calculated/modeled, and experimental + calc.
In crystallography, the progress of refinement from crystal structures is monitored via different  factors. One example is the factor based on
factors. One example is the factor based on  :
:
 .
.
 factors can be used to describe the quality of the crystal data, but should be handled with care [14].
factors can be used to describe the quality of the crystal data, but should be handled with care [14].
Source of Crystal Data
The ball and stick figures are derived from crystal structures found via CSD. The CCDC refcodes for each structure used are listed in the Demonstration.
References
[1] J-P. Sauvage and C. Dietrich-Buchecker, Molecular Catenanes, Rotaxanes, and Knots: A Journey through the World of Molecular Topology, New York: Wiley-VCH, 1999.
[2] C. Wolf, Dynamic Stereochemistry of Chiral Compounds, Cambridge, UK: Royal Society of Chemistry, 2008.
[3] E. V. Anslyn and D. A. Dougherty, Modern Physical Organic Chemistry, Sausalito, CA: University Science Books, 2006.
[4] J. Loren, P. Gantzel, A. Linden, and J. Siegel, "Synthesis of Achiral and Racemic Catenanes Based on Terpyridine and a Directionalized Terpyridine Mimic, Pyridyl-phenanthroline," Organic & Biomolecular Chemistry, 3(17), 2005 pp. 3105–3116. doi:10.1039/B506101F.
[5] C. McArdle, S. Van, M. Jennings, and R. Puddephatt, "Gold(I) Macrocycles and Topologically Chiral [2]Catenanes," Journal of the American Chemical Society, 124(15), 2002 pp. 3959–3956. doi:10.1021/ja012006+.
[6] N. Fu, J. Baumes, E. Arunkumar, B. Noll, and B. Smith, "Squaraine Rotaxanes with Boat Conformation Macrocycles," The Journal of Organic Chemistry, 74(17), 2009 pp. 6462–6468. doi:10.1021/jo901298n.
[7] K. Nikitin and H. Müller-Bunz, "Encapsulation of 4,4´-bipyridinium Cations by Two Crown Ether Molecules: Formation and Structure of [3]pseudorotaxanes," New Journal of Chemistry, 33, 2009 pp. 2472–2478. doi:10.1039/B9NJ00414A.
[8] J. Bulier, A. Jouaiti, N. Kyritsakas-Gruber, L. Allouche, J-M. Planeix, and M. Hosseini, "Direct Synthesis and Structural Characterisation of Tri- and Tetra-nuclear Silver Metallaknotanes by Self-Assembly Approach," Chemical Communications, 46, 2008 pp. 6191–6193. doi:10.1039/B815580A.
[9] J-F. Ayme, J. Beves, D. Leigh, R. McBurney, K. Rissanen, and D. Schultz, "A Synthetic Molecular Pentafoil Knot," Nature Chemistry, 4, 2012 pp. 15–20. doi:10.1038/nchem.1193.
[10] S. Tokuji, J-Y. Shin, K. Kim, J. Lim, K. Youfu, S. Saito, D. Kim, and A. Osuka, "Facile Formation of a Benzopyrane-Fused [28]Hexaphyrin that Exhibits Distinct Möbius Aromaticity," Journal of the American Chemical Society, 131(21), 2009 pp. 7240–7241. doi:10.1021/ja902836x.
[11] J. Sankar, S. Mori, S. Saito, H. Rath, M. Suzuki, Y. Inokuma, H. Shinokubo, K. Kim, Z. Yoon, J-Y. Shin, J. Lim, Y. Matsuzaki, O. Matsushita, A. Muranaka, N. Kobayashi, D. Kim, and A. Osuka, "Unambiguous Identification of Möbius Aromaticity for meso-Aryl-Substituted [28]Hexaphyrins(1.1.1.1.1.1)," Journal of the American Chemical Society, 130(41), 2008 pp. 13568–13579. doi:10.1021/ja801983d.
[12] R. Herges, "Topology in Chemistry: Designing Möbius Molecules," Chemical Reviews, 106(12), 2006 pp. 4820–4842. doi:10.1021/cr0505425.
[13] C. Pentecost, A. Peters, K. Chichak, G. Cave, S. Cantrill, and J. Stoddart, "Chiral Borromeates," Angewandte Chemie, 118(25), 2006 pp. 4205–4210. doi:10.1002/ange.200600817.
[14] International Union of Crystallography. "Online Dictionary Of Crystallography: R Factor." (Apr 4, 2008) reference.iucr.org/dictionary/R_factor.
Permanent Citation