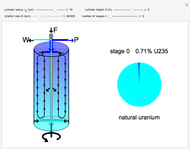
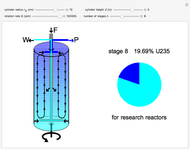
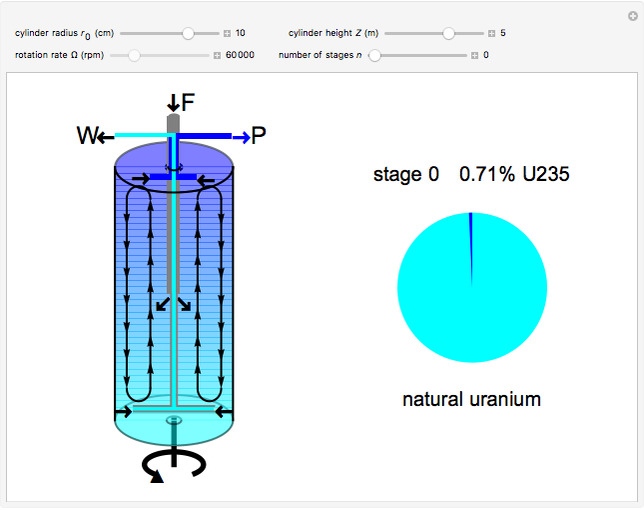
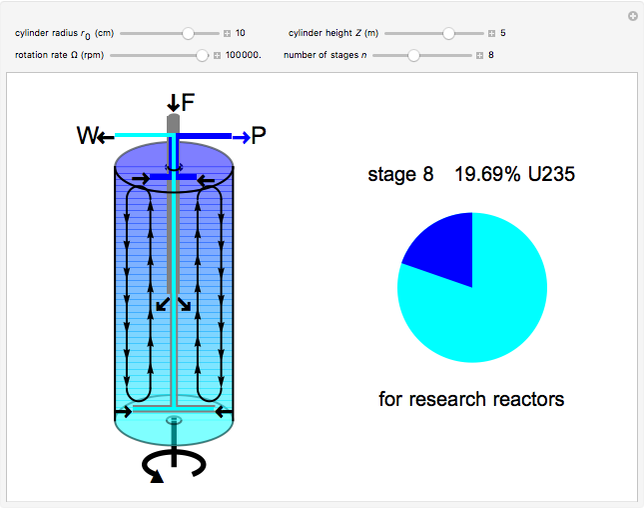
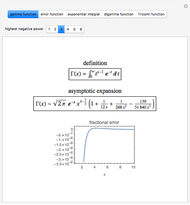
Natural uranium has an isotopic composition consisting of 99.284% U238 and 0.711% U235 (plus a very small fraction of U234) [1–4]. Only the U235 isotope can undergo nuclear fission for use in nuclear reactors and weapons. For nuclear power reactors, the uranium must be enriched to approximately 3 to 5% U235. Some research reactors use enrichment to 12–20%, while nuclear weapons require at least 85% enrichment.
The most prevalent method for uranium enrichment makes use of a cascade of gas centrifuges. Uranium hexafluoride  sublimes into a gas, referred to as "hex," at 56 ˚C (
sublimes into a gas, referred to as "hex," at 56 ˚C ( K) under normal atmospheric pressure. Fortunately, fluorine is isotopically pure
K) under normal atmospheric pressure. Fortunately, fluorine is isotopically pure  , so that the mass difference between
, so that the mass difference between  (
( ) and
) and  (
( ) is due entirely to the uranium isotopes. This Demonstration describes a simplified model of the Zippe centrifuge, developed in the years following World War II.
) is due entirely to the uranium isotopes. This Demonstration describes a simplified model of the Zippe centrifuge, developed in the years following World War II.
Gas molecules in a rotating cylinder experience a centrifugal force pushing them toward the outer wall of the cylinder. The density distribution has a form analogous to the barometer formula, for the atmospheric density in a gravitational field, specifically,
 ,
,
where  is density,
is density,  is the distance from the axis of the cylinder,
is the distance from the axis of the cylinder,  is the angular velocity of rotation,
is the angular velocity of rotation,  is the absolute temperature and
is the absolute temperature and  is the gas constant. The local mole fraction of U235 is given by
is the gas constant. The local mole fraction of U235 is given by
 ,
,
where  kg/mol.
kg/mol.
Typical rotor spin rates are in excess of 60,000 rpm, which corresponds to  . With cylinder radii of the order of 10 cm, almost all the gas is flattened into a narrow shell clinging to the cylinder walls, known as the Stewartson layer.
. With cylinder radii of the order of 10 cm, almost all the gas is flattened into a narrow shell clinging to the cylinder walls, known as the Stewartson layer.
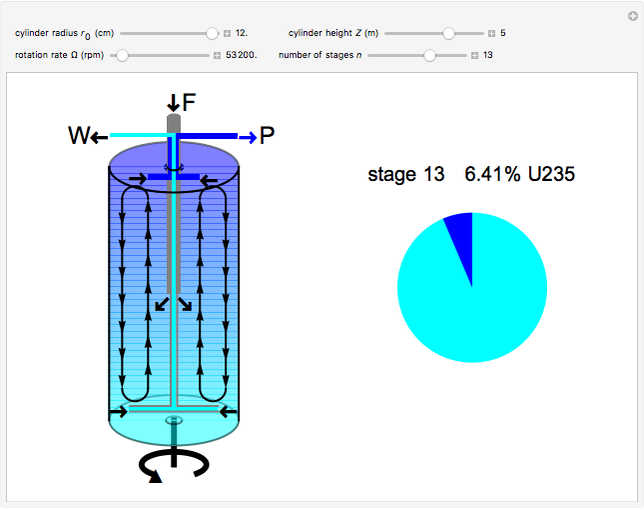
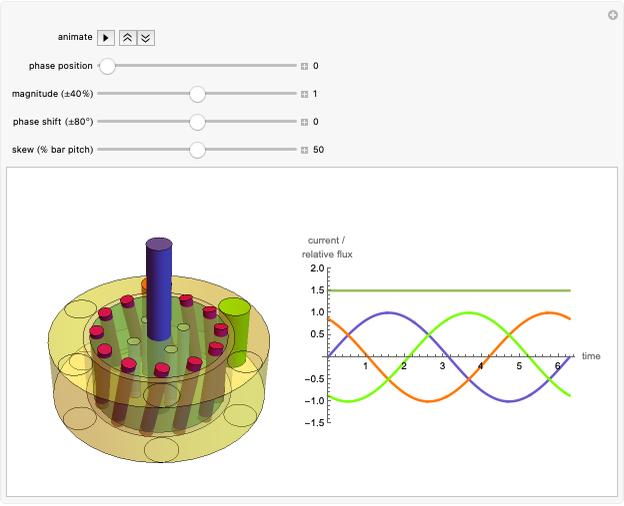
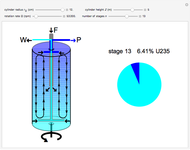
The success of the gas centrifuge is made possible by changing the direction of enrichment from radial to axial by inducing vertical circulation of the hex between the top and bottom of the cylinder. Convection in the gas is produced by creating a temperature gradient by heating the bottom of the cylinder. As a result of the vertical circulation, the enriched hex can be withdrawn from the top of the centrifuge, while the depleted hex is drawn out from the bottom.
A single centrifuge can achieve a separation factor in the range of 1.2 to 1.5, meaning that the fraction of U235 is enhanced (from its beginning value of 0.007) by this factor. Clearly, to produce the enrichment required for reactor use, a cascade of connected centrifuges must be used, with the enriched output from one stage fed into a successive stage. The end product might require as many as 20 stages. There are usually multiple banks of centrifuges for each stage, to increase the total output of enriched product (see photo in Details).
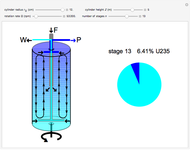
The graphic is a highly idealized and simplified representation of a gas centrifuge. Hex at some intermediate stage of enrichment is fed into the inlet F, while the outlets P and W withdraw the product (U235 enriched hex) and the waste (U235 depleted hex), respectively.
The pie chart on the right shows the isotopic composition of the uranium after  centrifuge stages.
centrifuge stages.
[less]