Orbital Transformations in Diels-Alder Reaction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
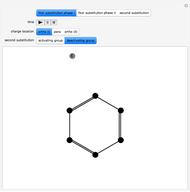
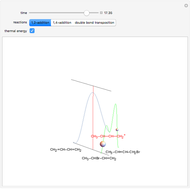
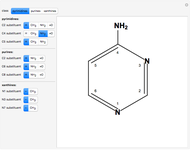
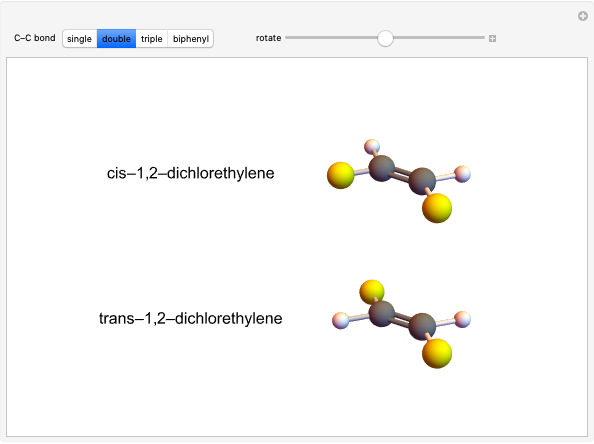
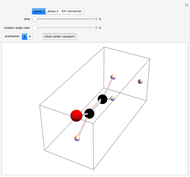
The Diels–Alder reaction is a [4+2]-cycloaddition of a conjugated diene (such as butadiene) and a dienophile (such as ethylene):
[more]
Contributed by: S. M. Blinder (September 2018)
Open content licensed under CC BY-NC-SA
Snapshots
Details
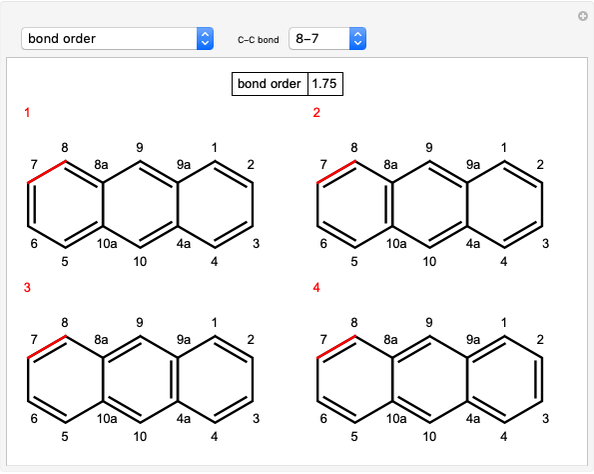
The significance of frontier orbitals, HOMOs and LUMOs, in the reactivity of aromatic hydrocarbons was first recognized by Kenichi Fukui in 1952. Frontier orbitals are analogous to valence electrons for atoms.
The Diels–Alder reaction conforms to the conservation of orbital symmetry, a set of principles proposed by Woodward and Hoffmann in 1965. In a concerted reaction, the molecular orbitals of the starting materials are transformed into the molecular orbitals of the products in a smooth, continuous way. Stereospecificity of the substituents on all carbon centers is preserved, which is important in the synthesis of complex molecules, particularly natural products.
References
[1] Wikipedia. "Diels–Alder Reaction." (Sep 7, 2018) en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction.
[2] Wikipedia. "Frontier Molecular Orbital Theory." (Sep 7, 2018) en.wikipedia.org/wiki/Frontier_molecular_orbital _theory.
[3] Wikipedia. "Woodward–Hoffmann Rules." (Sep 7, 2018) en.wikipedia.org/wiki/Woodward%E2%80%93Hoffmann_rules.
Permanent Citation