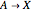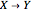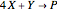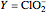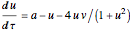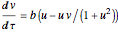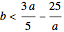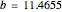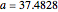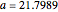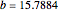Chlorite-Iodide-Malonic Acid (CIMA) Reaction
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
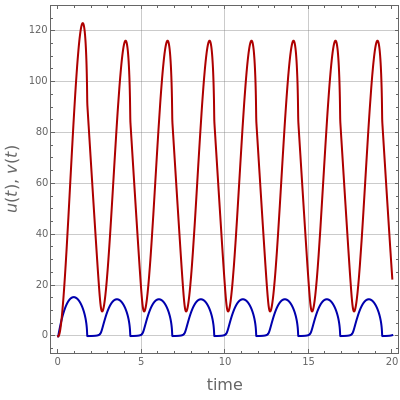
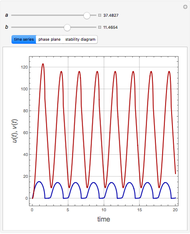
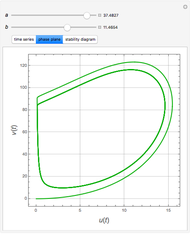
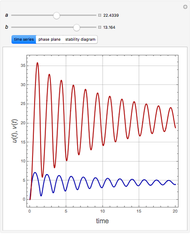
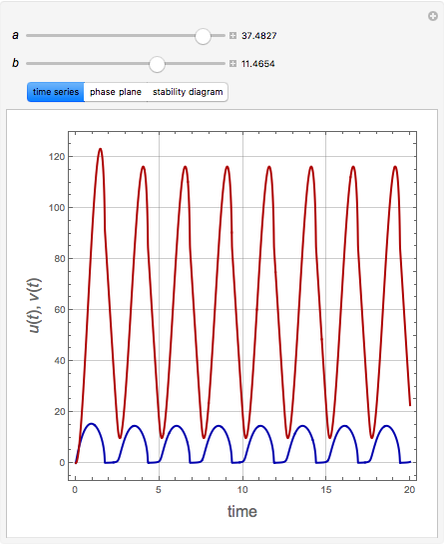
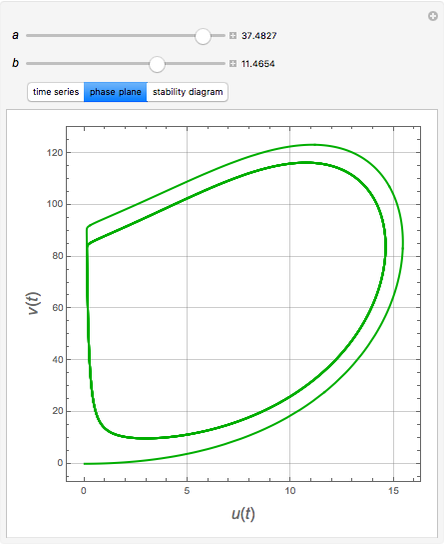
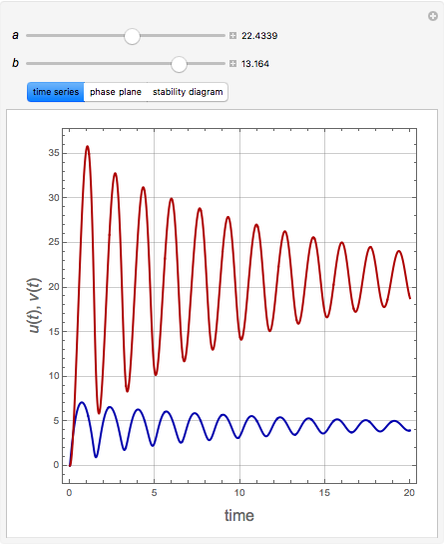
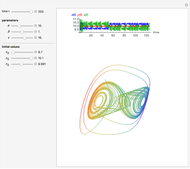
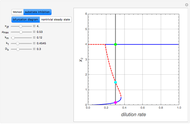
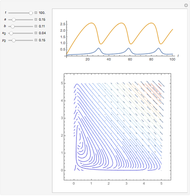
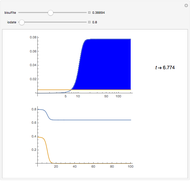
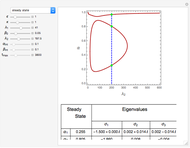
The Demonstration presents the chlorite-iodide-malonic acid (CIMA) reaction, which gives rise to temporal oscillations.
[more]
Contributed by: Housam Binous, Brian G. Higgins, and Ahmed Bellagi (January 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] I. R. Epstein and J. A. Pojman, An Introduction to Nonlinear Chemical Dynamics: Oscillations, Waves, Patterns, and Chaos, New York: Oxford University Press, 1998.
[2] G. Nicolis, Dynamique chimique: thermodynamique, cinétique et mécanique statistique, Paris: Dunod, 2005.
Permanent Citation