Conversion of Methanol to Formaldehyde

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
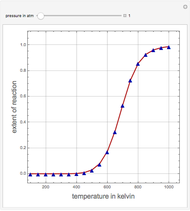
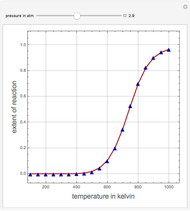
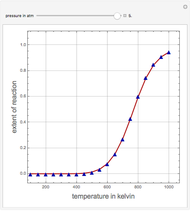
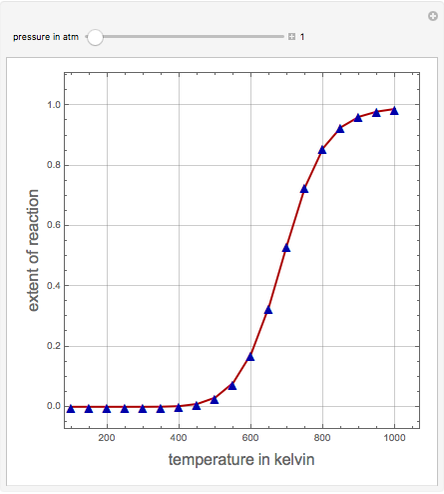
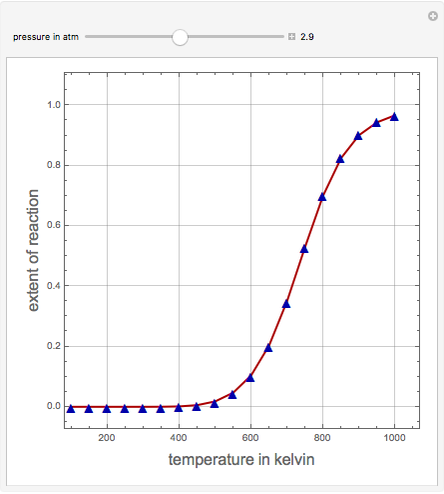
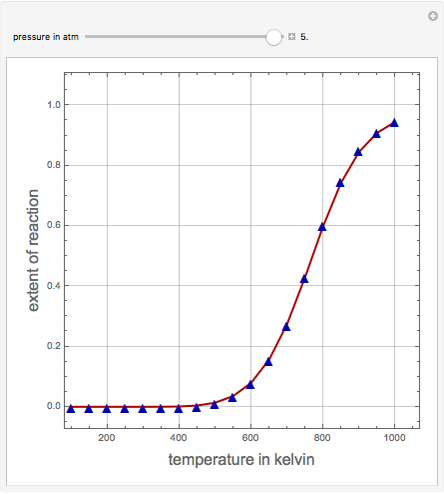
Consider the production of formaldehyde by gas-phase pyrolysis of methanol [1] by the reaction  . This Demonstration plots the extent of the reaction versus temperature (in kelvin) for user-set values of the pressure
. This Demonstration plots the extent of the reaction versus temperature (in kelvin) for user-set values of the pressure  . Two methods are compared: (1) the Gibbs free energy minimization technique, shown by the red curve; and (2) the reaction coordinate method, indicated by the blue triangles. Perfect agreement is observed between the two approaches.
. Two methods are compared: (1) the Gibbs free energy minimization technique, shown by the red curve; and (2) the reaction coordinate method, indicated by the blue triangles. Perfect agreement is observed between the two approaches.
Contributed by: Housam Binous, Mohammad Mozahar Hossain, and Ahmed Bellagi (March 2016)
(King Fahd University of Petroleum & Minerals, KSA; ENIM, University of Monastir, Tunisia)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] M. D. Koretsky, Engineering and Chemical Thermodynamics, 2nd ed., Hoboken, NJ: Wiley, 2013.
Permanent Citation
"Conversion of Methanol to Formaldehyde"
http://demonstrations.wolfram.com/ConversionOfMethanolToFormaldehyde/
Wolfram Demonstrations Project
Published: March 14 2016