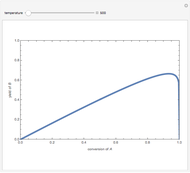
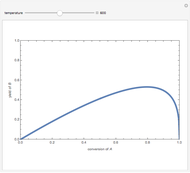
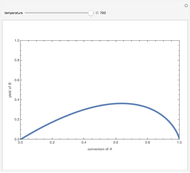
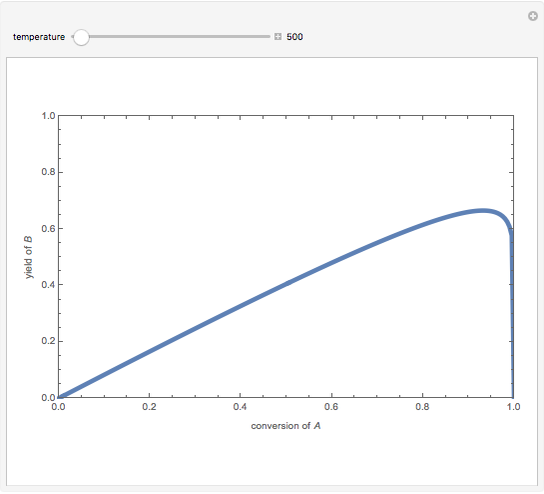
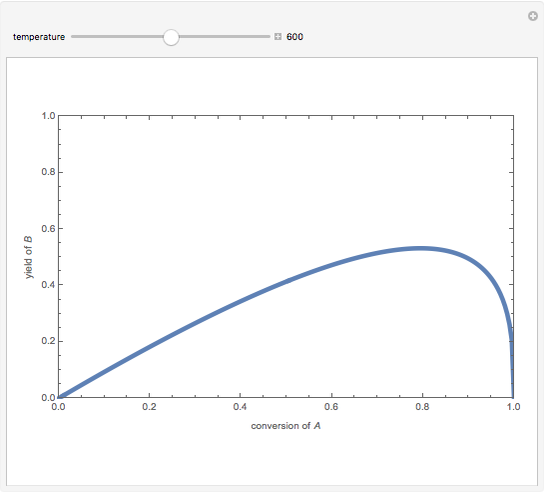
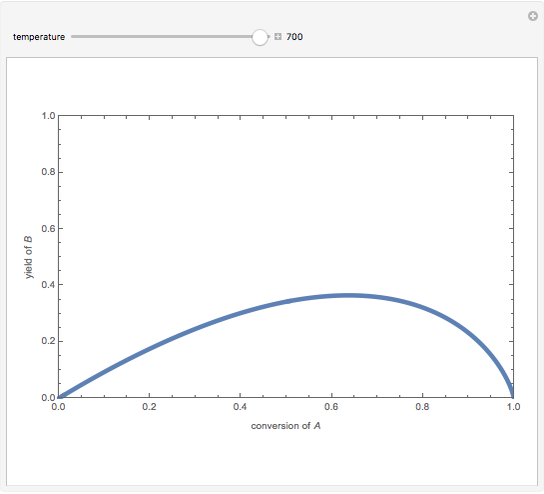
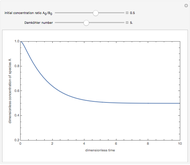
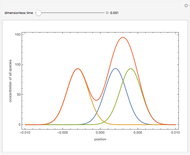
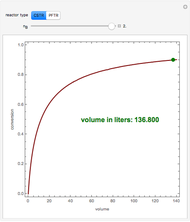
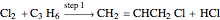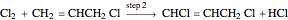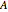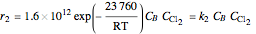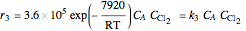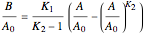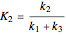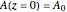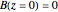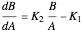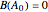Isothermal Yield of Allyl Chloride versus Conversion of Propylene
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Consider the gas-phase chlorination of propylene with 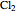 to produce allyl chloride. The reaction network is as follows:
to produce allyl chloride. The reaction network is as follows:
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
J. J. Carberry, Chemical and Catalytic Reaction Engineering, Mineola, NY: Dover, 2001.
Permanent Citation