De Novo Growth Processes with Competing Mechanisms

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
This Demonstration depicts the evolution of spontaneous peaked processes and chemical reactions such as acrylamide or peroxides formation and degradation where the initial number or concentration is zero.
Contributed by: Mark D. Normand, Maria G. Corradini, and Micha Peleg (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
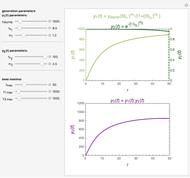
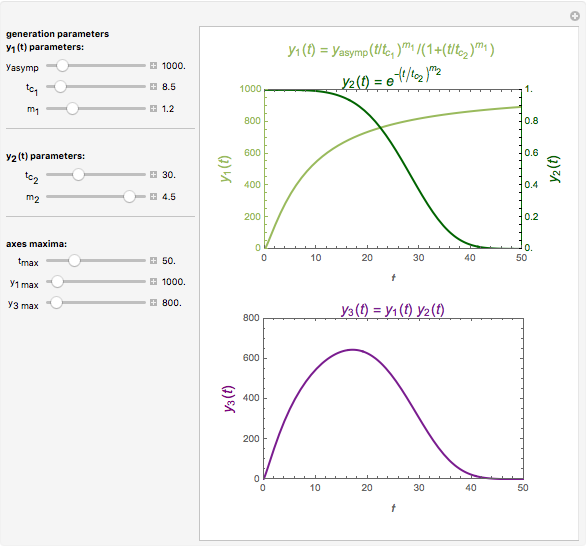
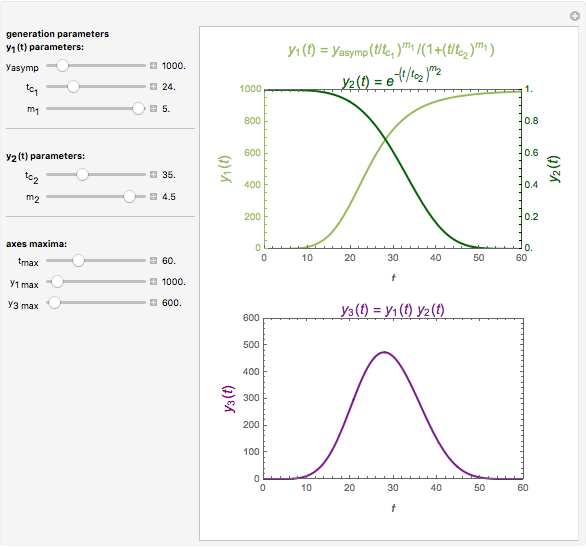
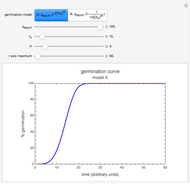
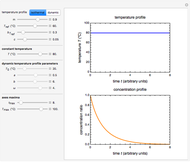
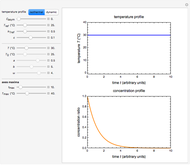
Snapshot 1: monotonic concentration increase
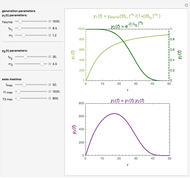
Snapshot 2: a prominent peak with no lag and final concentration approaching zero
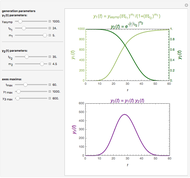
Snapshot 3: a prominent peak appearing after a lag with final concentration approaching zero
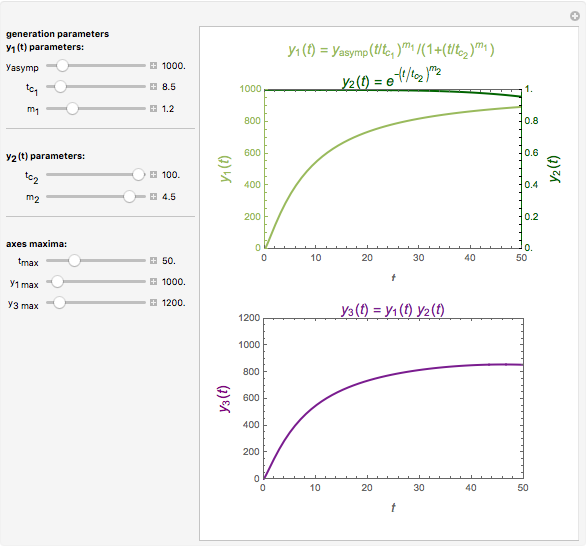
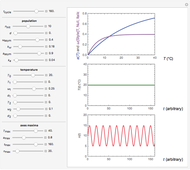
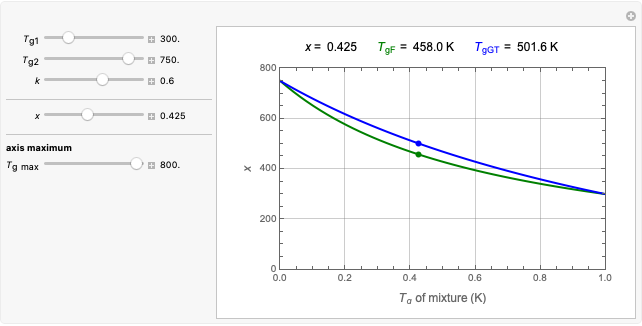
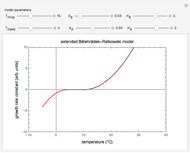
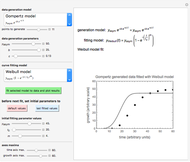
This Demonstration depicts processes or chemical reactions starting at zero initial concentration. The process or reaction is governed by two competing mechanisms, one of growth or generation and the other of mortality or degradation. The concentration, 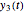 , is the product of the growth component,
, is the product of the growth component, 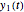 , representing the system's potential for uninhibited growth, and a decay factor,
, representing the system's potential for uninhibited growth, and a decay factor, 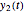 ,
, 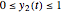 , which represents the decay component. Each has a characteristic time scale,
, which represents the decay component. Each has a characteristic time scale, 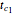 and
and 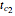 , and "rate parameter",
, and "rate parameter", 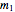 and
and 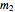 , whose values along with
, whose values along with 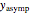 can be modified using sliders.
can be modified using sliders. 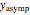 is the maximum possible concentration that could be attained had no degradation taken place. The Demonstration plots the functions
is the maximum possible concentration that could be attained had no degradation taken place. The Demonstration plots the functions 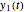 and
and 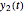 together in green and the corresponding
together in green and the corresponding 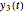 in purple. The maxima of the
in purple. The maxima of the  ,
, 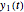 , and
, and 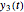 axes can also be adjusted with sliders. The equations for
axes can also be adjusted with sliders. The equations for 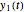 ,
, 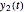 , and
, and 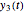 appear above the plots.
appear above the plots.
Permanent Citation
"De Novo Growth Processes with Competing Mechanisms"
http://demonstrations.wolfram.com/DeNovoGrowthProcessesWithCompetingMechanisms/
Wolfram Demonstrations Project
Published: March 7 2011