Residue Curve in a Ternary Equilateral Diagram

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
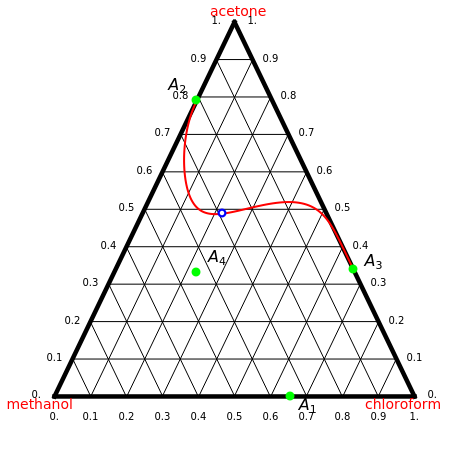
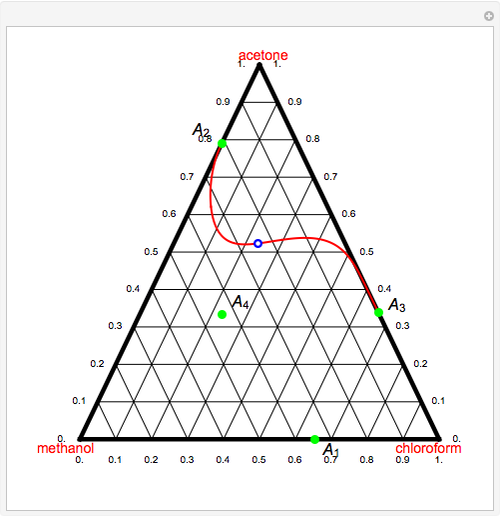
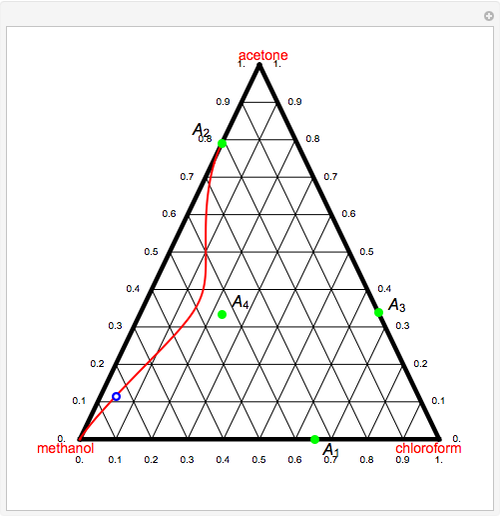
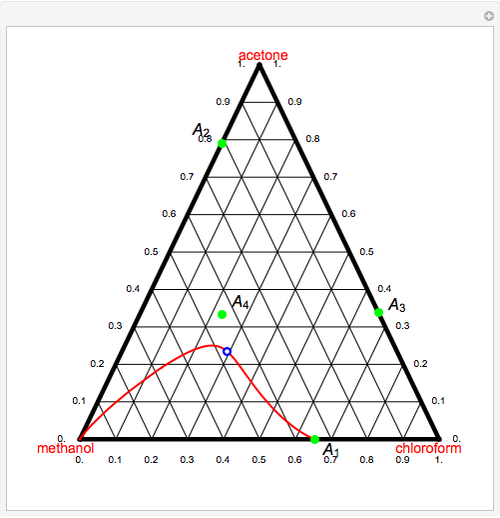
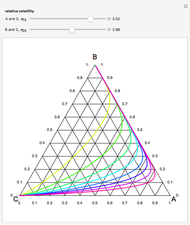
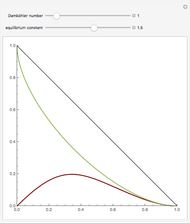
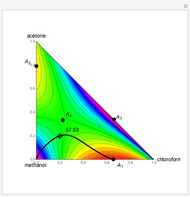
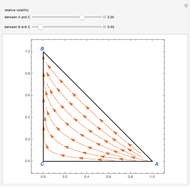
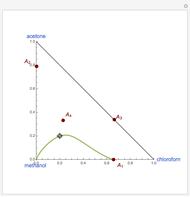
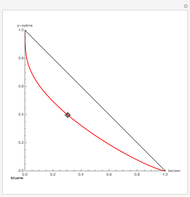
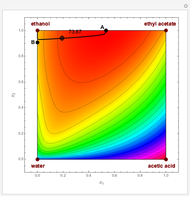
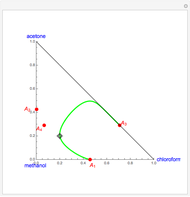
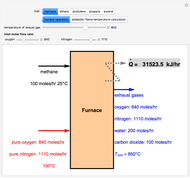
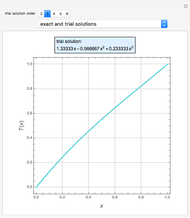
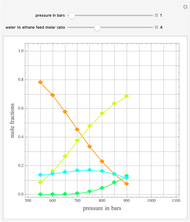
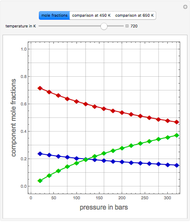
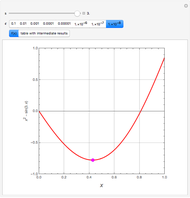
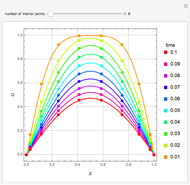
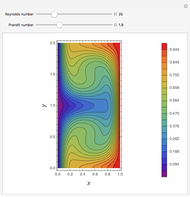
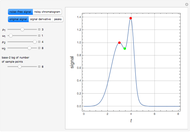
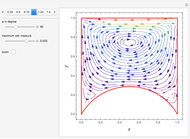
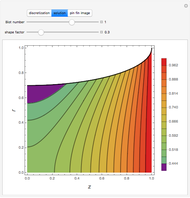
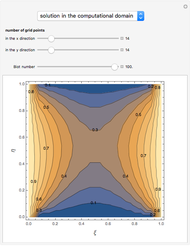
Consider a ternary mixture of chloroform, acetone, and methanol at  . The vapor-liquid equilibrium (VLE) behavior is described by the modified Raoult's law with activity coefficients predicted by the Wilson model [1].
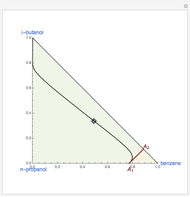
This mixture presents four azeotropes, whose locations and boiling points are:
. The vapor-liquid equilibrium (VLE) behavior is described by the modified Raoult's law with activity coefficients predicted by the Wilson model [1].
This mixture presents four azeotropes, whose locations and boiling points are:

Contributed by: Housam Binous and Brian G. Higgins (August 2011)
Open content licensed under CC BY-NC-SA
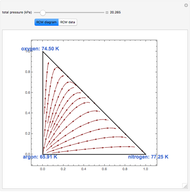
Snapshots
Details
Reference
[1] G. M. Wilson, "Vapor-Liquid Equilibrium XI: A New Expression for the Excess Free Energy of Mixing," Journal of the American Chemical Society, 86(2), 1964 pp. 127–130.
Permanent Citation