Spectral Series of the Hydrogen Atom

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
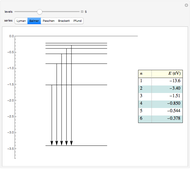
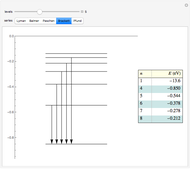
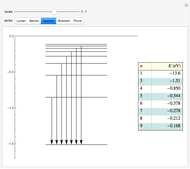
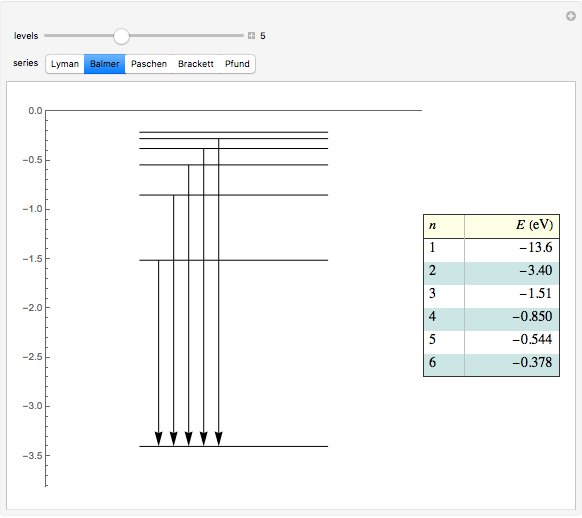
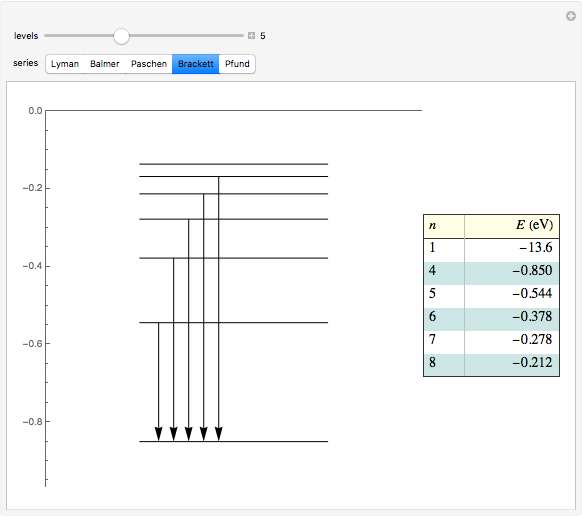
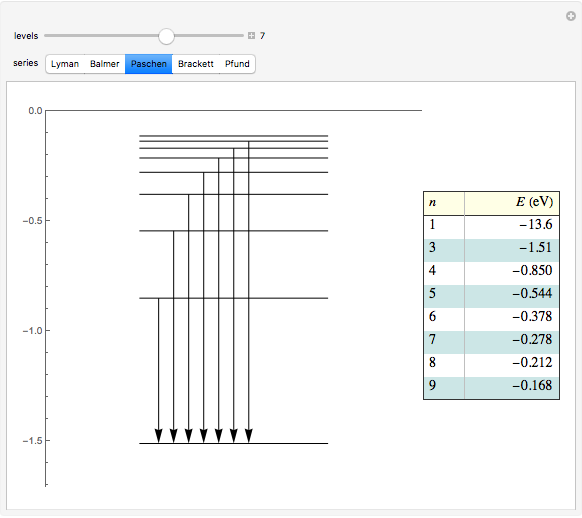
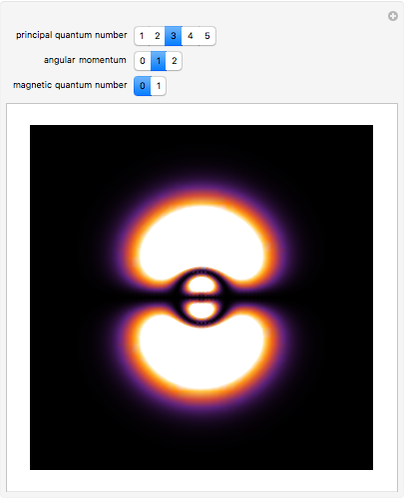
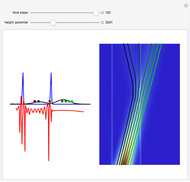
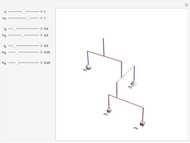
This diagram of the energy levels of the hydrogen atom shows the allowed transitions between them. The  ground state at -13.6 eV is not shown, nor are sublevels for each principal quantum number
ground state at -13.6 eV is not shown, nor are sublevels for each principal quantum number  . The energies are listed at the right and the quantum numbers are on the left. The first series of spectral lines was discovered by Johan Jacob Balmer in 1885, followed by other series named after their discoverers.
. The energies are listed at the right and the quantum numbers are on the left. The first series of spectral lines was discovered by Johan Jacob Balmer in 1885, followed by other series named after their discoverers.
Contributed by: Enrique Zeleny (January 2009)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Spectral Series of the Hydrogen Atom"
http://demonstrations.wolfram.com/SpectralSeriesOfTheHydrogenAtom/
Wolfram Demonstrations Project
Published: January 29 2009