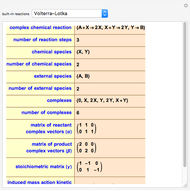
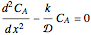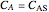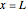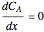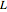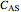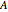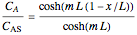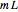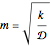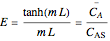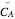Surface Kinetics with Pore Diffusion Resistance
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
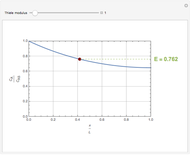
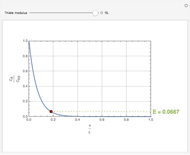
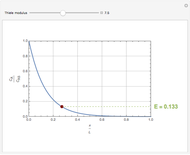
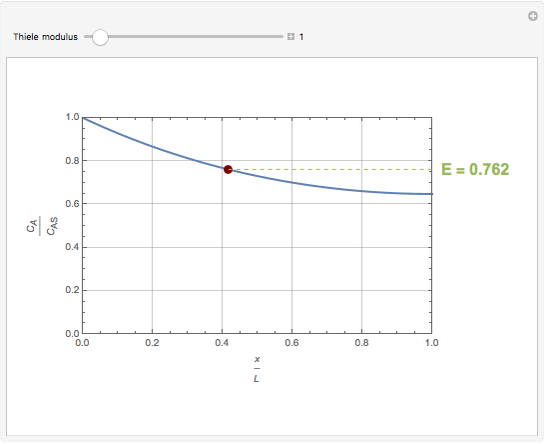
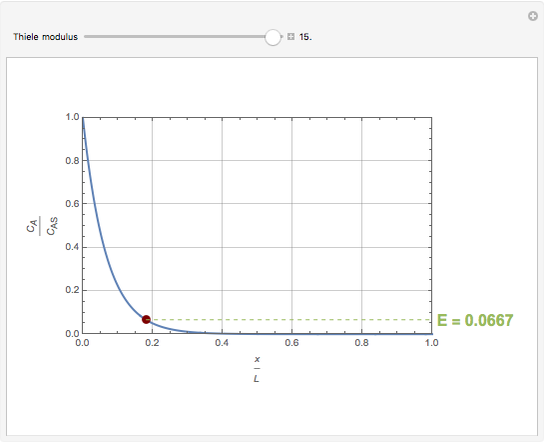
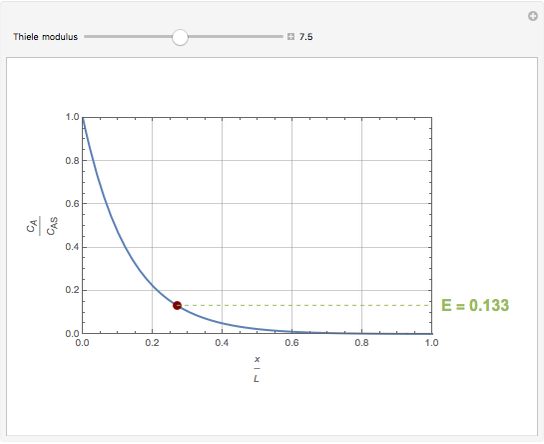
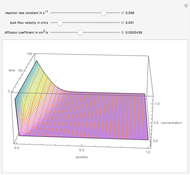
Consider a first order reaction 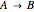 taking place on the surface of a catalyst with cylindrical pores. The relevant equation is
taking place on the surface of a catalyst with cylindrical pores. The relevant equation is
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
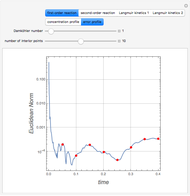
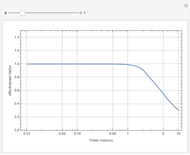
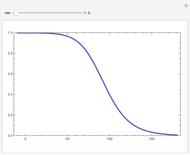
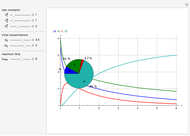
Snapshots
Details
O. Levenspiel, Chemical Reaction Engineering, 3rd ed., New York: John Wiley & Sons, 1999.
Permanent Citation