Compressibility Factor Charts

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
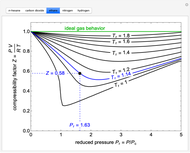
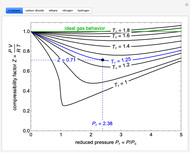
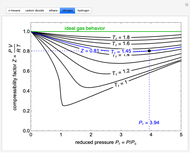
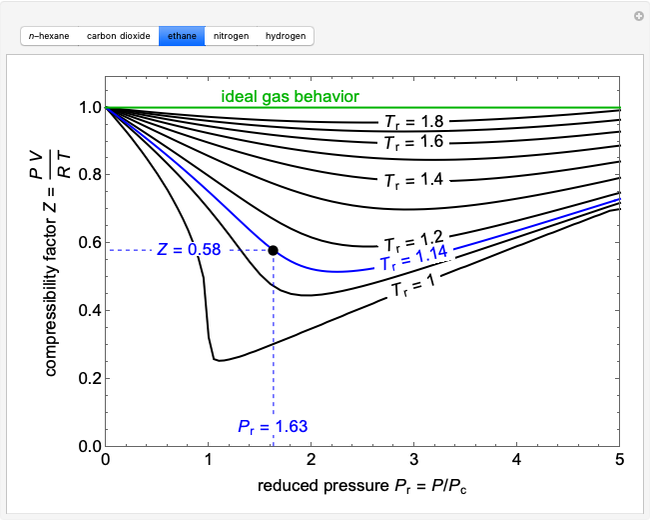
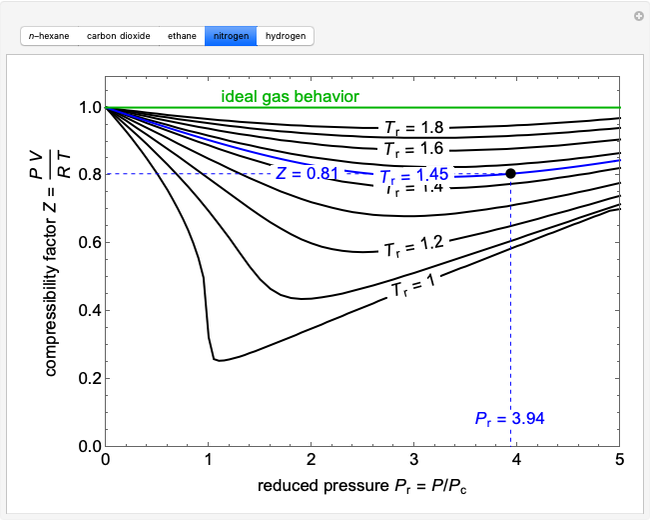
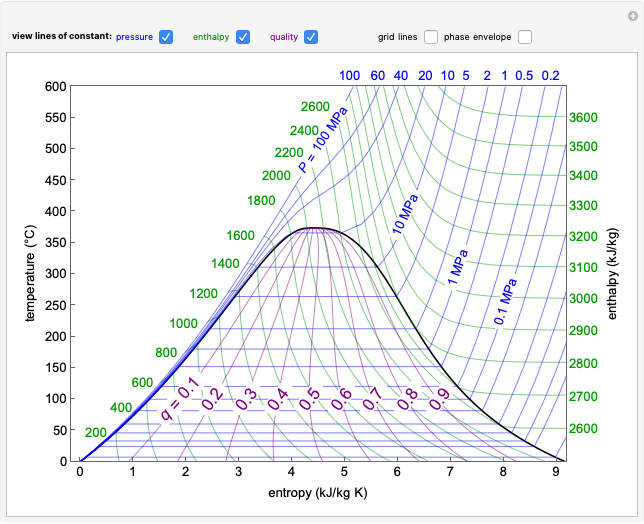
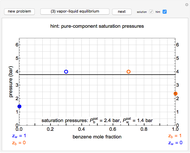
The compressibility factor chart plots the compressibility factor 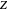 , equal to
, equal to 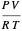 , where
, where 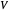 is the volume per mole, versus the reduced pressure
is the volume per mole, versus the reduced pressure 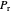 for several values of the reduced temperature
for several values of the reduced temperature 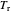 . The reduced pressure and temperature are defined by
. The reduced pressure and temperature are defined by 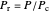 and
and 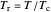 , respectively, where
, respectively, where 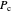 is the critical pressure and
is the critical pressure and 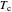 is the critical temperature. Use buttons to select one of five molecules, and move the black dot to display the compressibility factor curve (blue) for any value of
is the critical temperature. Use buttons to select one of five molecules, and move the black dot to display the compressibility factor curve (blue) for any value of 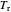 between 1.0 and 1.8. The
between 1.0 and 1.8. The 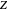 and
and 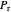 values for the location of the black dot are displayed on the chart. For an ideal gas,
values for the location of the black dot are displayed on the chart. For an ideal gas, 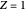 (green line).
(green line).
Contributed by: Rachael L. Baumann (May 2017)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The Soave modification of the Redlich–Kwong (SRK) equation of state is used to calculate the compressibility factor:
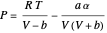 .
.
This equation can be written in terms of the reduced temperature 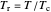 :
:
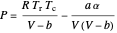 ,
,
where 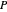 is pressure (bar),
is pressure (bar), 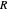 is the ideal gas constant (
is the ideal gas constant (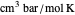 ),
), 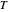 is temperature (K),
is temperature (K), 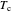 is the critical temperature (K),
is the critical temperature (K), 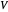 is volume (
is volume (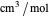 ), and
), and 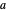 ,
,  and
and 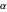 are constants:
are constants:
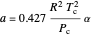 ,
,
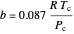 ,
,
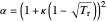 ,
,
where 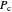 is the critical pressure
is the critical pressure 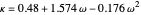 , and
, and 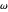 is the acentric factor.
is the acentric factor.
The compressibility factor is defined by:
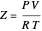 .
.
Permanent Citation