Detailed Analysis of Rutherford Scattering of Alpha Particles

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
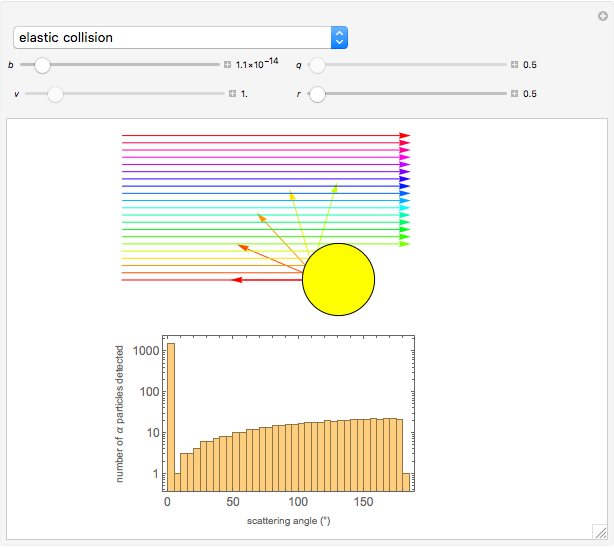
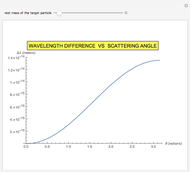
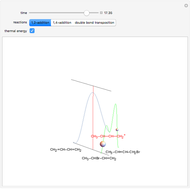
This Demonstration is intended to show how Rutherford, Marsden and Geiger managed to identify the presence of compact positive charges in atoms by interpreting the data from  particles scattering from the nuclei in a thin foil.
particles scattering from the nuclei in a thin foil.
Contributed by: D. Meliga and S. Z. Lavagnino (February 2017)
Additional contributions by: G. Follo,A. Ratti and G. Valorio
Open content licensed under CC BY-NC-SA
Snapshots
Details
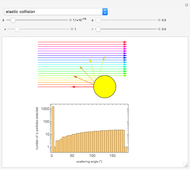
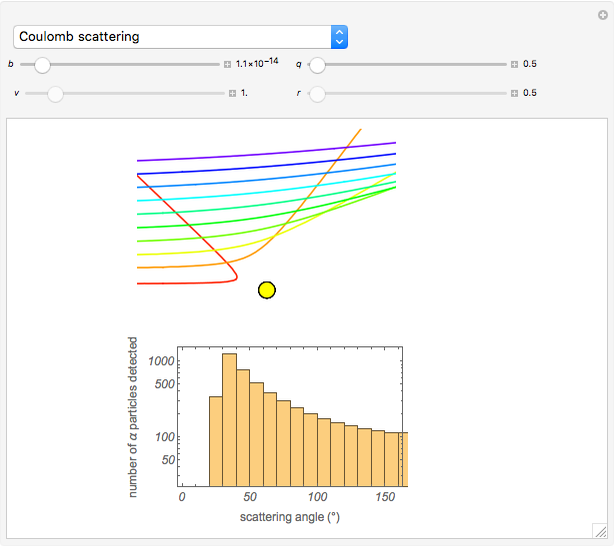
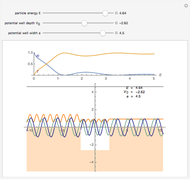
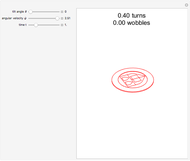
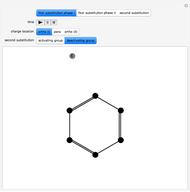
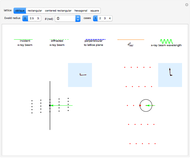
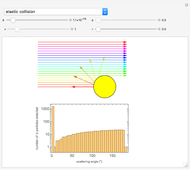
Snapshot 1: the hypothetical collision of  particles with a rigid sphere and the resulting scattering
particles with a rigid sphere and the resulting scattering
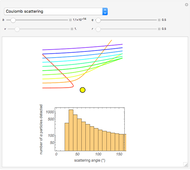
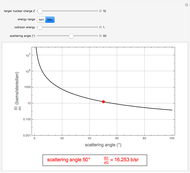
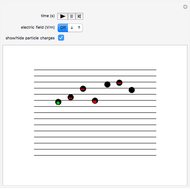
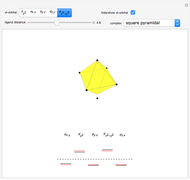
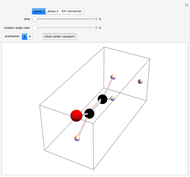
Snapshot 2: Coulomb interaction of  particles with a nucleus
particles with a nucleus
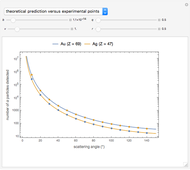
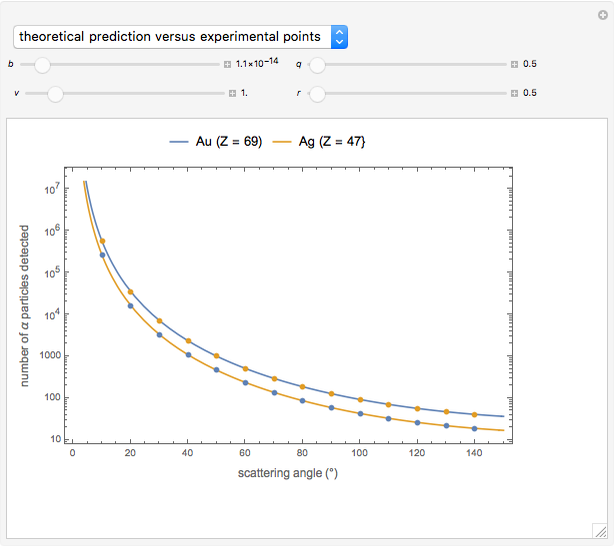
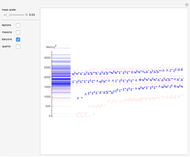
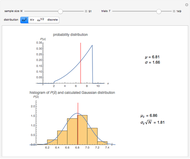
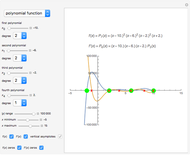
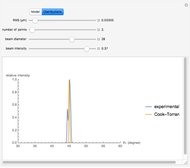
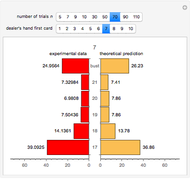
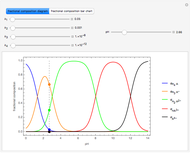
Snapshot 3: comparison of theory and experiment for  particle scattering from silver and gold foils [1, 3, 5]
particle scattering from silver and gold foils [1, 3, 5]
References
[1] R. A. Serway, C. J. Moses and C. A. Moyer, Modern Physics, 2nd ed., Fort Worth: Saunders College Publishers, 1997.
[2] R. I. Hulsizer. The Rutherford Atom [Video]. (Feb 15, 2017) www.youtube.com/watch?v=-DU9W9SsL_w&index=11&list=PLhAAV-jkgAdxUtFDMFlqZ-UwtgSWTz-i5.
[3] D. Halliday, R. Resnick and J. Walker, Fundamentals of Physics, 9th ed., Hoboken, NJ: John Wiley & Sons, 2011.
[4] M. S. Suzuki, "Rutherford Scattering and Hyperbola Orbit," ResearchGate, June 2016. www.researchgate.net/publication/303983573.
[5] E. Rutherford, J. Chadwick and C. D. Ellis, Radiations from Radioactive Substances, Cambridge, UK: Cambridge University Press, 1930.
Permanent Citation