Electrophilic Aromatic Substitution Reactions of Benzene

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
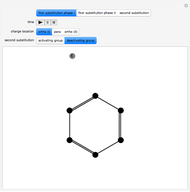
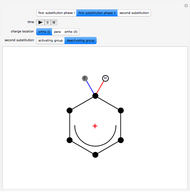
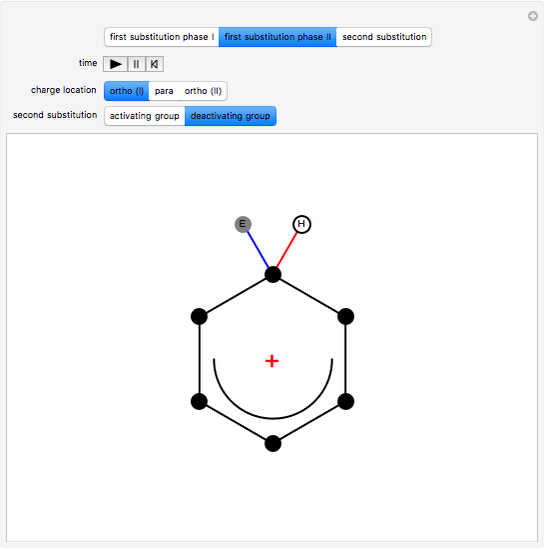
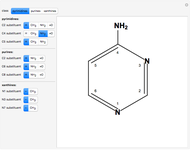
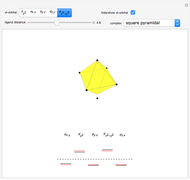
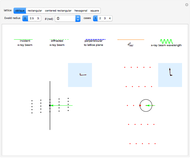
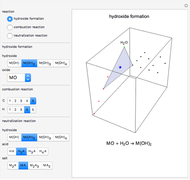
This Demonstration shows a common class of reactions of benzene: electrophilic substitutions. In the first substitution, phase I, displaced electrons on the aromatic ring attract an electrophilic species  , positively charged in almost all the cases. A carbocation is formed, stabilized by resonance contributions with increased positive charge in the ortho and para positions. In the first substitution phase II, protons,
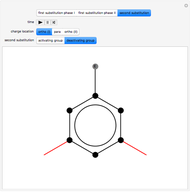
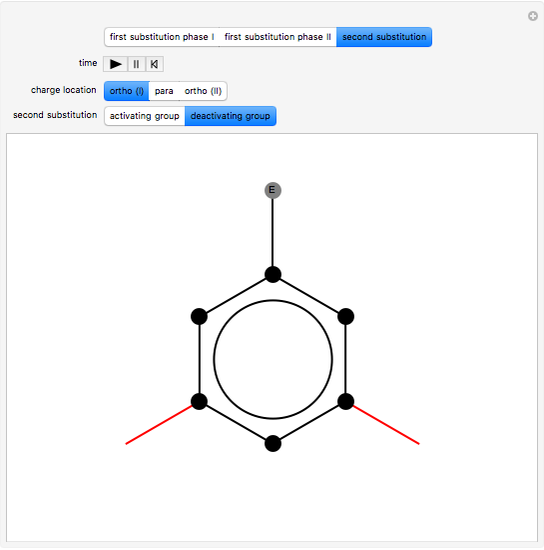
, positively charged in almost all the cases. A carbocation is formed, stabilized by resonance contributions with increased positive charge in the ortho and para positions. In the first substitution phase II, protons,  , are removed from the ring, thus restoring charge neutrality and aromaticity. Benzene is a planar molecule, with all of its bonds represented in black. The other bonds in the carbocation above and below the plane are colored in blue and red.
, are removed from the ring, thus restoring charge neutrality and aromaticity. Benzene is a planar molecule, with all of its bonds represented in black. The other bonds in the carbocation above and below the plane are colored in blue and red.
Contributed by: D. Meliga and S. Z. Lavagnino (January 2018)
Open content licensed under CC BY-NC-SA
Snapshots
Details
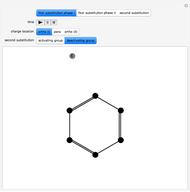
Snapshot 1: in the first phase, benzene undergoes attack from an electrophilic species
Snapshot 2: in the second phase, the carbocation loses the hydrogen at the point of the attack
Snapshot 3: the result of the second substitution is determined by the first substitution
Reference
[1] H. Hart, L. E. Craine and D. J. Hart, Organic Chemistry: A Short Course, 10th ed., Boston: Houghton Mifflin, Co., 1999.
Permanent Citation
"Electrophilic Aromatic Substitution Reactions of Benzene"
http://demonstrations.wolfram.com/ElectrophilicAromaticSubstitutionReactionsOfBenzene/
Wolfram Demonstrations Project
Published: January 19 2018