Infrared and Raman Vibrational Spectra of Methane

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
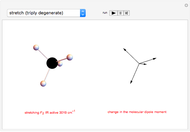
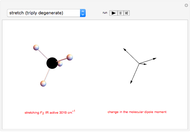
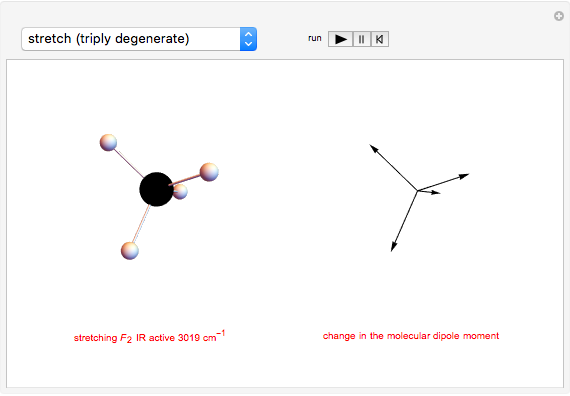
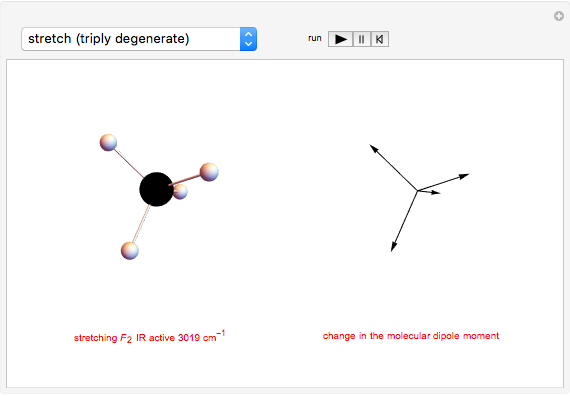
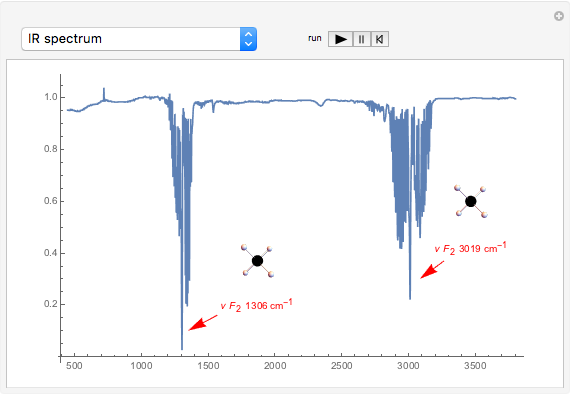
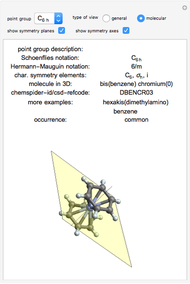
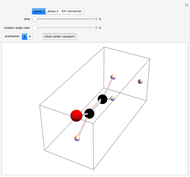
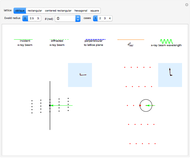
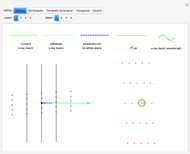
This Demonstration shows how vibrational transitions in a methane molecule  ) are associated with changes in its bond dipole moments. An IR-active vibrational transition occurs only when the vectorial sum of the individual C-H bond dipoles undergoes a change. If there is no such change in the dipole moment, the vibration is IR inactive, but still possibly active in the Raman spectrum. Each type of vibration is classified according to the symmetry group of the molecule [1].
) are associated with changes in its bond dipole moments. An IR-active vibrational transition occurs only when the vectorial sum of the individual C-H bond dipoles undergoes a change. If there is no such change in the dipole moment, the vibration is IR inactive, but still possibly active in the Raman spectrum. Each type of vibration is classified according to the symmetry group of the molecule [1].
Contributed by: D. Meliga and S. Z. Lavagnino (December 2016)
Additional contributions by: G. Follo and A. Ratti
Open content licensed under CC BY-NC-SA
Snapshots
Details
A nonlinear molecule with  atoms has
atoms has  vibrational degrees of freedom. For methane (
vibrational degrees of freedom. For methane ( ),
),  , and there are nine vibrational modes.
, and there are nine vibrational modes.
Taking account of degeneracy, just four types of vibrations can take place. Degeneracy occurs when different vibrations have the same frequency. The first type of vibration is nondegenerate, while the third type has a twofold degeneracy, and both the second and fourth types have threefold degeneracies [2].
Snapshot 1: symmetric stretching leading to an IR-inactive vibration
Snapshot 2: asymmetric stretching leading to an IR-active vibration
Snapshot 3: IR rotational-vibrational spectrum
References
[1] C. E. Housecroft and A. G. Sharpe, Inorganic Chemistry, 2nd ed., New York: Pearson/Prentice Hall, 2005.
[2] D. A. McQuarrie and J. D. Simon, Physical Chemistry: A Molecular Approach, Sausalito, CA: University Science Books, 1997.
Permanent Citation