Electrostatic Bonding Forces between Atoms

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
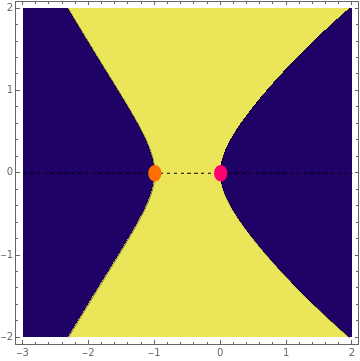
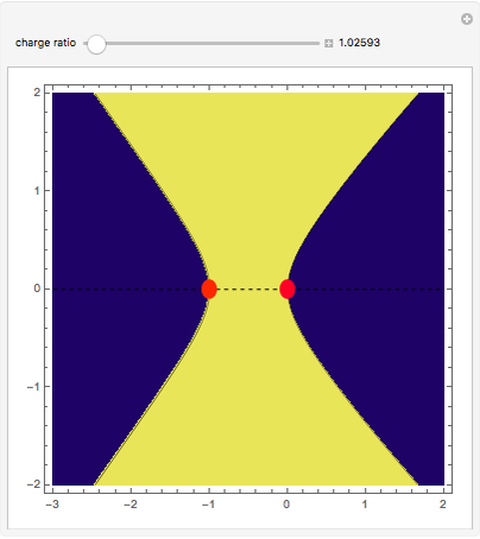
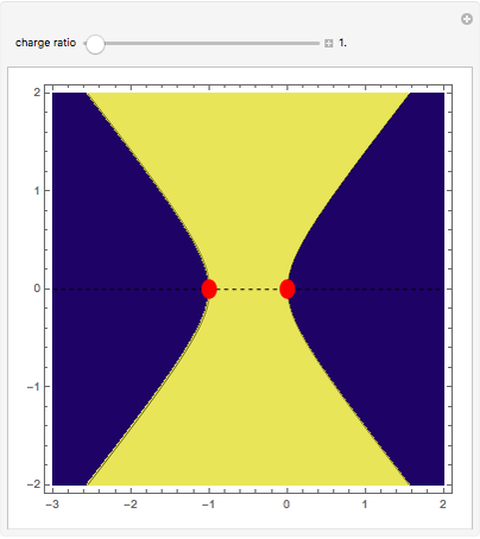
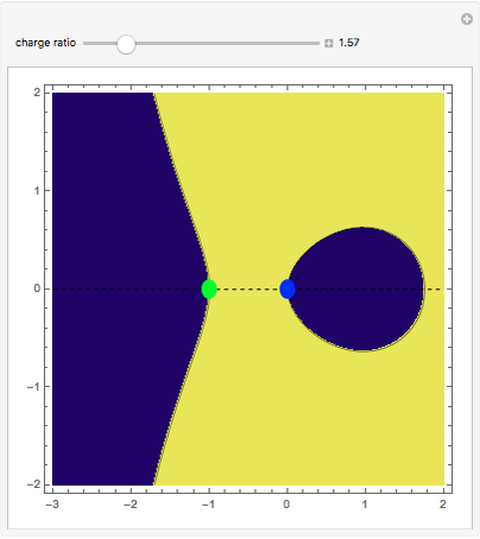
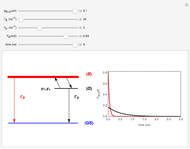
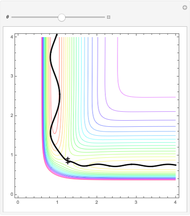
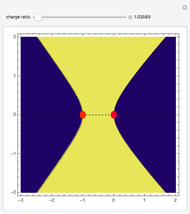
This Demonstration examines bonding forces by placing a charge between two separated ions. The contour represents the curve where the repulsive and attractive forces are counterbalanced by the charge. Changing the charge ratio  lets you examine heteronuclear (
lets you examine heteronuclear ( ) to ionic (
) to ionic ( ) species.
) species.
Contributed by: Eric R. Bittner,Department of Chemistry, University of Houston (March 2011)
Open content licensed under CC BY-NC-SA
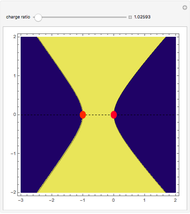
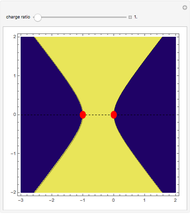
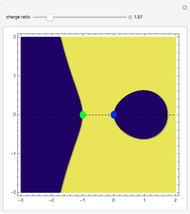
Snapshots
Details
Theoretical Details:
This Demonstration examines the electronic bonding forces between two ions A and B separated by a distance  along the
along the  axis. In the absence of any electrons, the repulsive force between the ions acting along the A-B axis would be
axis. In the absence of any electrons, the repulsive force between the ions acting along the A-B axis would be
 .
.
Now, suppose we put a single charge,  , at some point P in the
, at some point P in the  -
- plane. We then have two attractive forces
plane. We then have two attractive forces  and
and  between the ions and the point charge. If we consider the component of
between the ions and the point charge. If we consider the component of  along the A-B bond axis, we can define a bonding force,
along the A-B bond axis, we can define a bonding force,
 ,
,
where 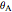 and
and  denote the angles PAB and PBA, respectively. In a molecule, the bonding force is then computed by taking the expectation value over the electronic state
denote the angles PAB and PBA, respectively. In a molecule, the bonding force is then computed by taking the expectation value over the electronic state  ; however, we can gain some insight into bonding by simply looking at the functional form of
; however, we can gain some insight into bonding by simply looking at the functional form of  . For this we follow the discussion in Barry, Rice, and Ross [1] that in turn is based upon ideas introduced by Kajans and Berlin [2]. This is also discussed in the classic book by Hirschfelder, Curtiss, and Bird [3].
. For this we follow the discussion in Barry, Rice, and Ross [1] that in turn is based upon ideas introduced by Kajans and Berlin [2]. This is also discussed in the classic book by Hirschfelder, Curtiss, and Bird [3].
First, whenever the charge is located between the two ions, there is an attractive force, since  and
and  , hence
, hence  . Likewise, if any charge is placed "behind" either ion, there is a destabilizing force, since
. Likewise, if any charge is placed "behind" either ion, there is a destabilizing force, since  . The curve where
. The curve where  is then the demarcation between the bonding and anti-bonding regions.
is then the demarcation between the bonding and anti-bonding regions.
The boundary surface given by
 ,
,
where  and
and  depends on only one physical parameter, the charge ratio
depends on only one physical parameter, the charge ratio  between the two ions. By varying this parameter you can see how bonding occurs between different ionic species.
between the two ions. By varying this parameter you can see how bonding occurs between different ionic species.  corresponds to any homonuclear diatomic species, while various heteronuclear species correspond to
corresponds to any homonuclear diatomic species, while various heteronuclear species correspond to  . For example,
. For example,  corresponds to NaCl and
corresponds to NaCl and  corresponds to HCl.
corresponds to HCl.
References [1] R. S. Berry, S. A. Rice, and J. Ross, Physical Chemistry, New York: Wiley, 1980. [2] K. Fajans and T. Berlin, "Quantization of Molecules, Inter- and Intramolecular Forces," Phys. Rev. 63(7-8), 1943 pp. 309–312. [3] J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, New York: Wiley, 1954.
Permanent Citation