Reactor Rate and Conversion versus Space Velocity

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
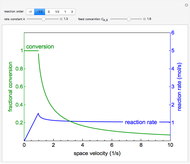
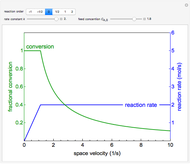
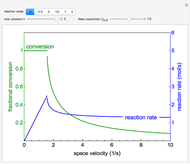
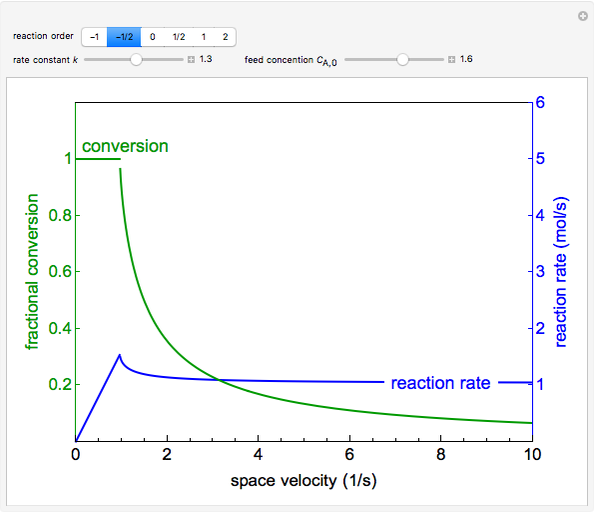
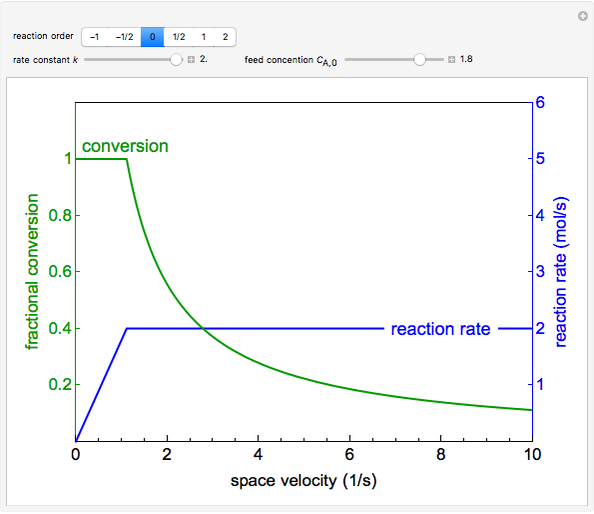
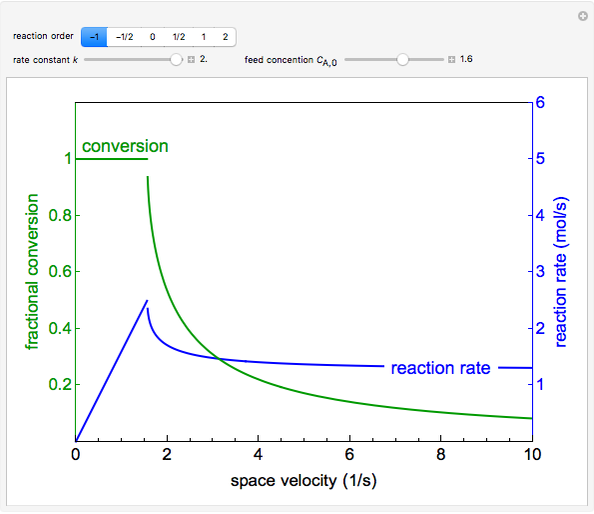
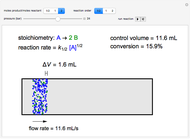
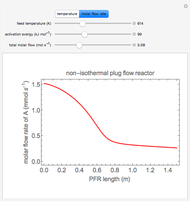
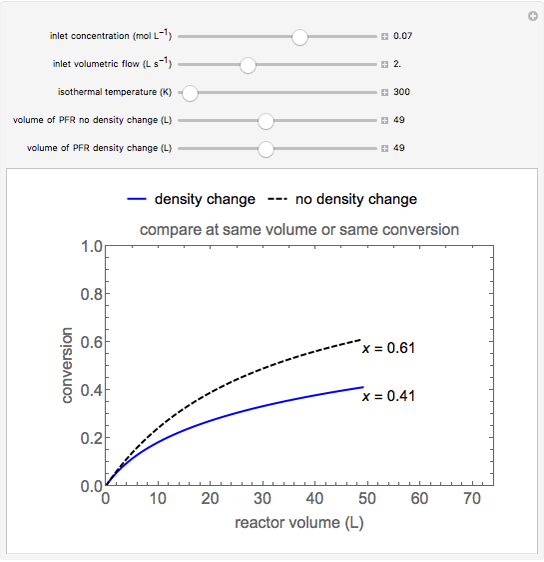
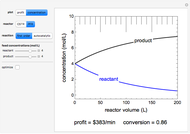
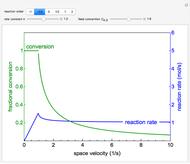
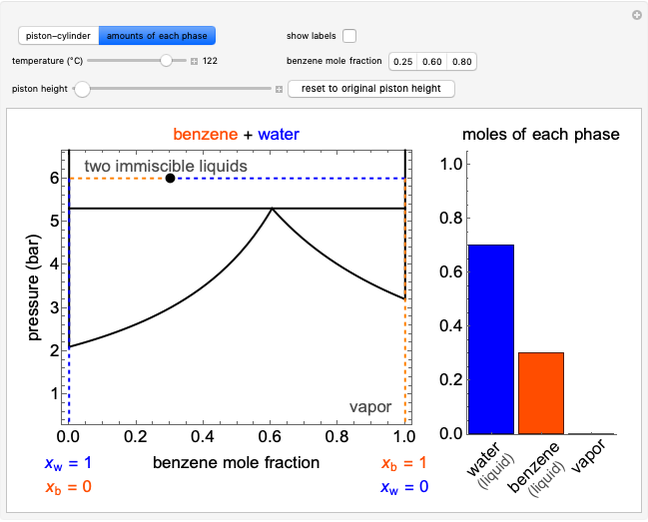
The irreversible reaction  takes place in an isothermal plug-flow reactor (PFR). The rate of reaction and the fractional conversion are plotted versus space velocity (entering volumetric flow rate/reactor volume). As space velocity increases, the time available for reaction decreases. Use sliders to vary the dimensionless rate constant and dimensionless feed concentration. Select the reaction order with buttons. Conversion is high for smaller space velocities, regardless of the order of reaction, and for a first-order reaction, conversion is independent of feed concentration.
takes place in an isothermal plug-flow reactor (PFR). The rate of reaction and the fractional conversion are plotted versus space velocity (entering volumetric flow rate/reactor volume). As space velocity increases, the time available for reaction decreases. Use sliders to vary the dimensionless rate constant and dimensionless feed concentration. Select the reaction order with buttons. Conversion is high for smaller space velocities, regardless of the order of reaction, and for a first-order reaction, conversion is independent of feed concentration.
Contributed by: Derek M. Machalek (June 2015)
Additional contributions by: Rachael L. Baumann, John L. Falconer, and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
For all reactions the instantaneous rate is  ,
,
where  is the change in the moles of
is the change in the moles of  with time,
with time,  is the kinetic constant,
is the kinetic constant,  is the concentration of
is the concentration of  , and
, and  is the order of the reaction.
is the order of the reaction.
The conversion is calculated from  ,
,
where  is the space velocity,
is the space velocity,  is the conversion of
is the conversion of  , and
, and  is the conversion of
is the conversion of  at the exit.
at the exit.
The conversion has an upper limit of 1, so that if 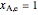 at a given space velocity, then conversion is 1 at lower space velocities.
at a given space velocity, then conversion is 1 at lower space velocities.
The overall rate is  ,
,
where  is the total number of moles reacting with time in the reactor, and
is the total number of moles reacting with time in the reactor, and  is the feed concentration of
is the feed concentration of  .
.
Negative first-order reaction:
instantaneous rate: 
conversion: 
negative half-order reaction:
instantaneous rate: 
conversion: 
zero-order reaction:
instantaneous rate: 
conversion: 
half-order reaction:
instantaneous rate: 
conversion: 
first-order reaction:
instantaneous rate: 
conversion: 
second-order reaction:
instantaneous rate: 
conversion: 
Permanent Citation