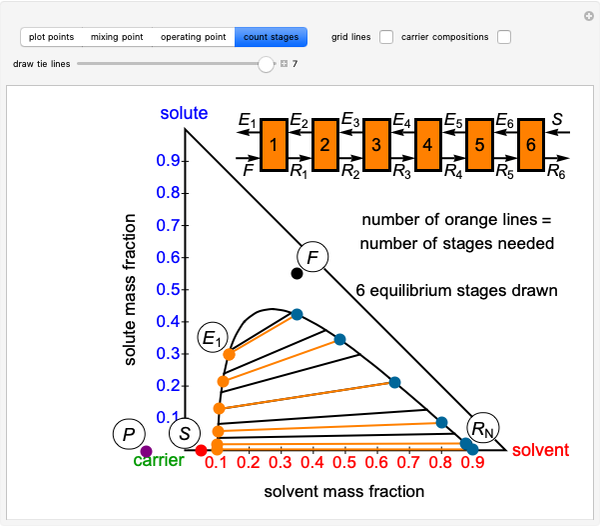
Temperature-Composition Diagram for Immiscible Liquids

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
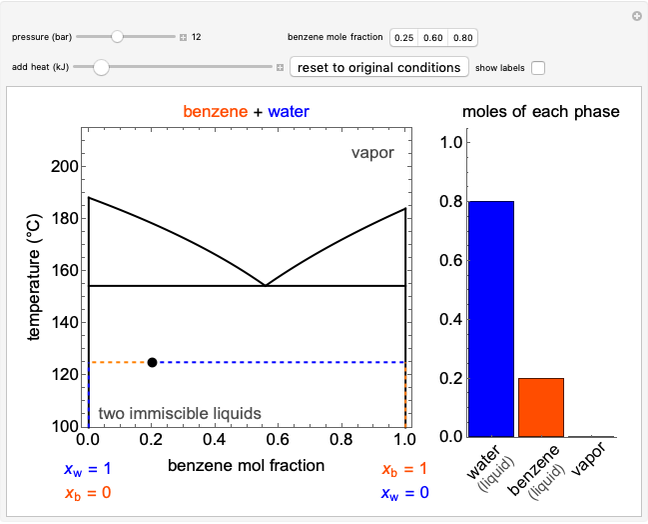
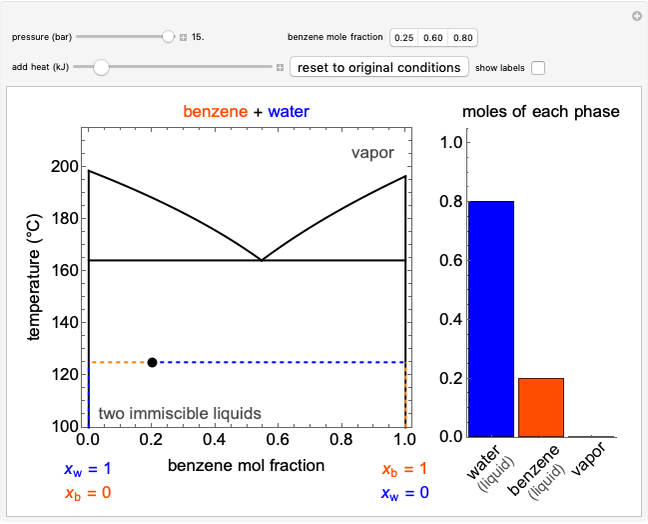
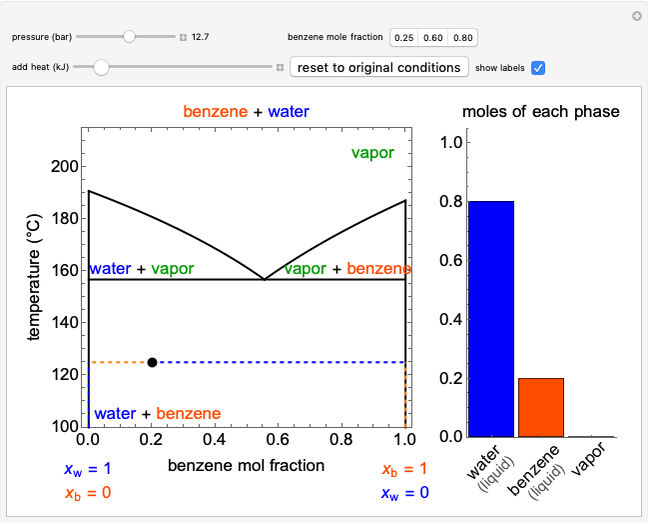
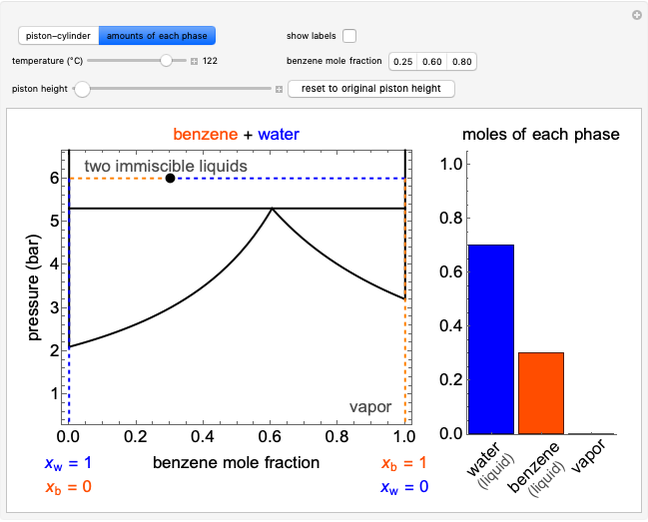
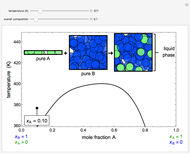
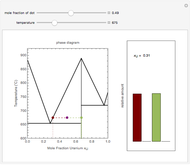
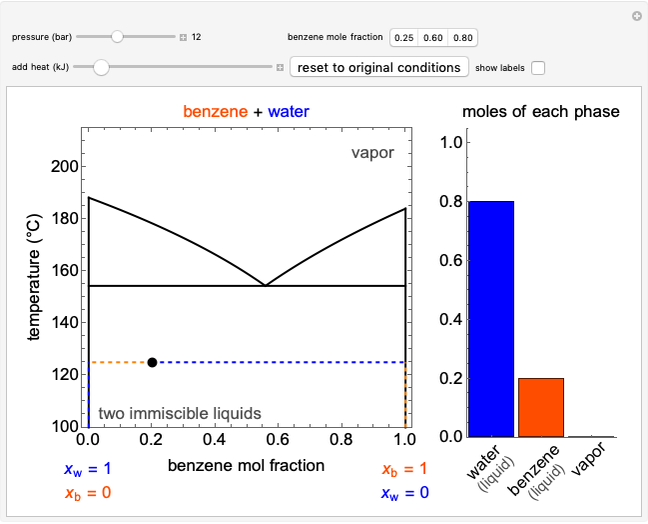
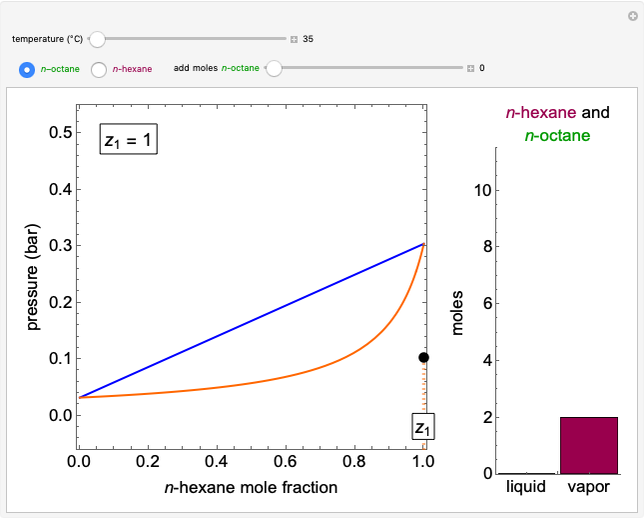
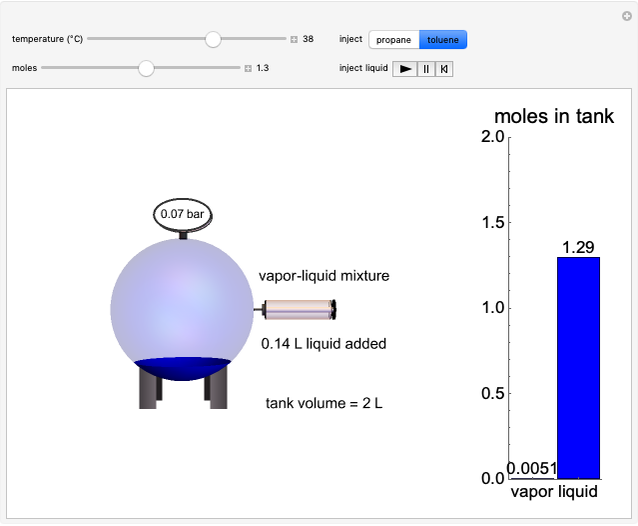
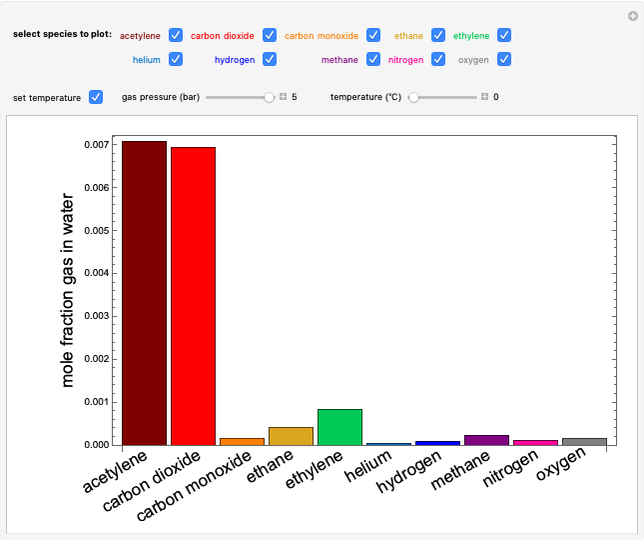
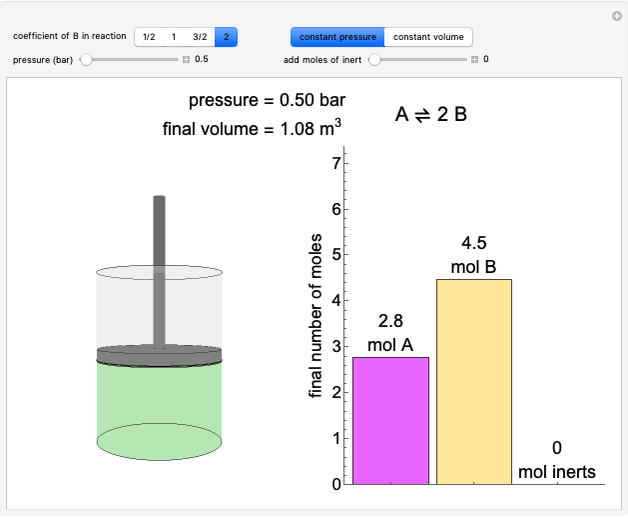
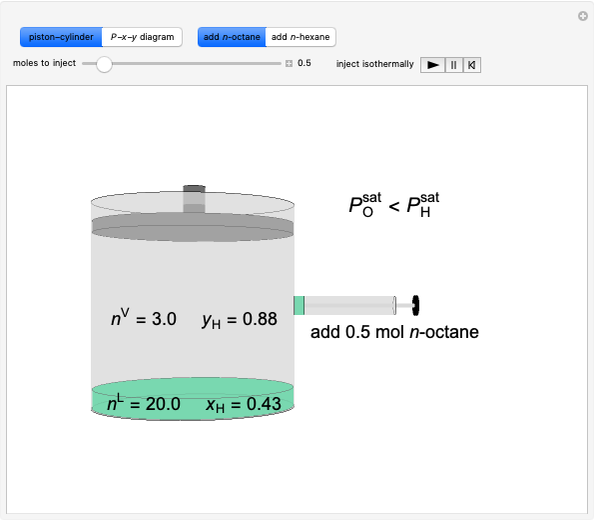
The temperature-composition phase diagram for two immiscible liquids, benzene and water, is at constant pressure. Set the pressure with a slider to change the saturation temperature. Set the overall benzene mole fraction with its slider. The bar graph shows the moles of liquid water (blue), liquid benzene (orange) and vapor (green), which contain both components. The system contains one mole total. You can add heat to change the temperature and the amounts in each phase. At a given pressure, all three phases co-exist at only one temperature. When heat is added at this temperature, one of the liquid phases completely evaporates before the temperature increases.
Contributed by: Rachael L. Baumann (April 2015)
Additional contributions by: John L. Falconer, Derek M. Machalek, and Garrison J. Vigil
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The pressure  of the vapor above two immiscible liquids, benzene and water, is the sum of the individual saturation pressures
of the vapor above two immiscible liquids, benzene and water, is the sum of the individual saturation pressures  (benzene) and
(benzene) and  (water):
(water):
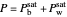 ,
,
The Antoine equation is used to calculate the saturation pressures:
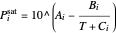 ,
,
where  ,
, 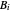 , and
, and 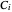 are Antoine constants for component
are Antoine constants for component  , and
, and  is temperature (°C).
is temperature (°C).
The dew point curves for the two liquids can be found by solving the following equations for temperature.
For conditions where liquid water is present:
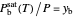 ,
,
and for conditions where liquid benzene is present:
 ,
,
where  and
and  are the vapor mole fractions for benzene and water.
are the vapor mole fractions for benzene and water.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Temperature-Composition Diagram for Immiscible Liquids. www.colorado.edu/learncheme/thermodynamics/TxyImmiscibleLiquids.html.
Permanent Citation