Solid-Solid-Liquid Equilibrium

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
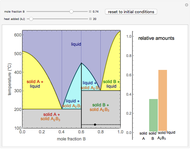
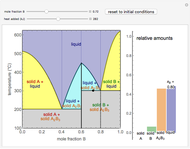
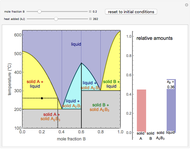
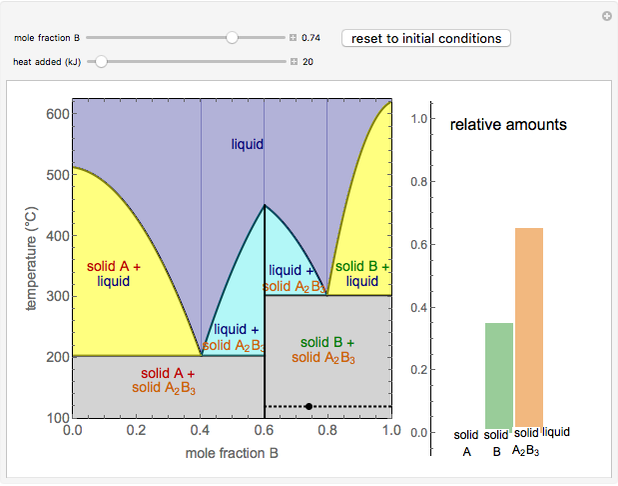
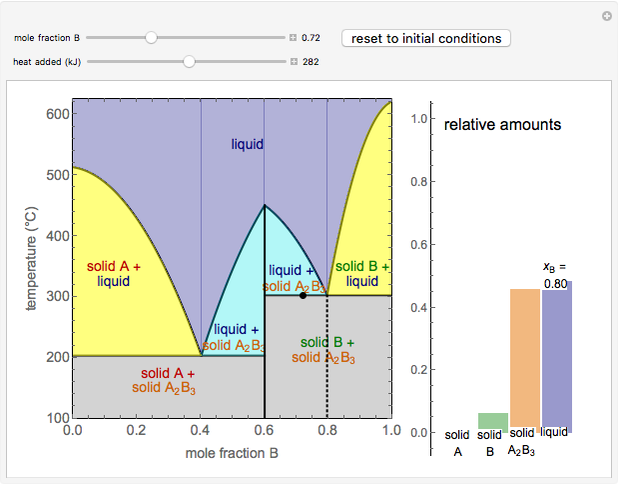
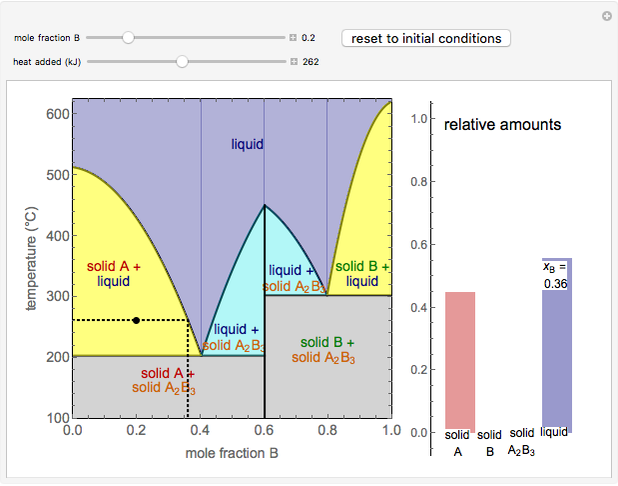
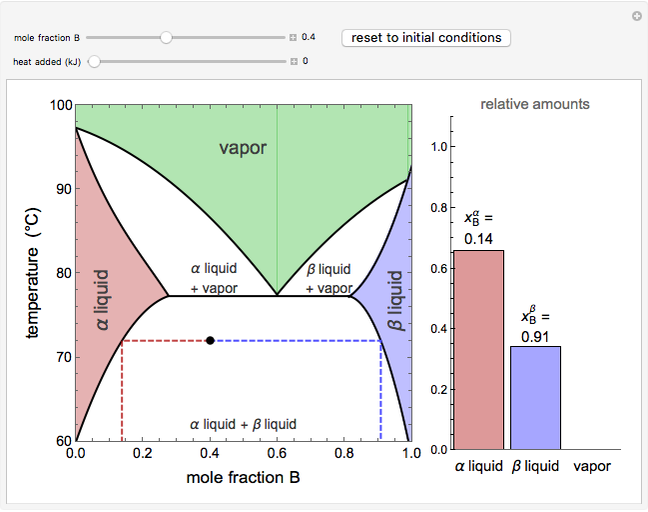
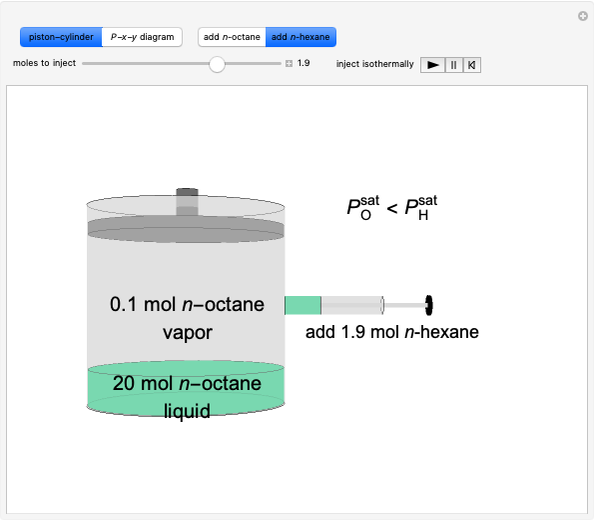
This Demonstration illustrates the behavior of a system of two pure substances (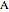 and
and 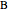 ) and a solid compound of the two (
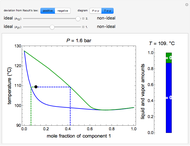
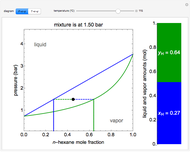
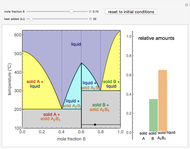
) and a solid compound of the two (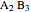 ). Solid-liquid equilibrium and solid-solid-liquid equilibrium are both represented in the phase diagram. Mixtures of pure solids are immiscible. The relative amounts of the four possible phases, given the temperature and mole fraction of
). Solid-liquid equilibrium and solid-solid-liquid equilibrium are both represented in the phase diagram. Mixtures of pure solids are immiscible. The relative amounts of the four possible phases, given the temperature and mole fraction of 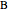 (represented by the black point in the
(represented by the black point in the 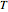 -
- -
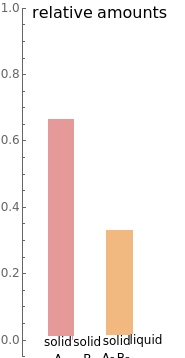
-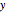 diagram), are shown in the bar graph to the right of the diagram. The mole fraction of
diagram), are shown in the bar graph to the right of the diagram. The mole fraction of 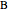 in the liquid phase (mixture of liquid
in the liquid phase (mixture of liquid 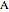 and liquid
and liquid 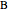 ) is given above the liquid bar in the bar graph. Since each solid phase is pure for the respective solid component, their mole fractions are omitted.
) is given above the liquid bar in the bar graph. Since each solid phase is pure for the respective solid component, their mole fractions are omitted.
Contributed by: Megan E. Maguire (November 2014)
Additional contributions by: Rachael L. Baumann
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
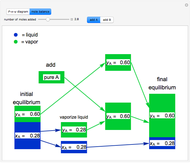
The lever rule is used to find the relative molar contents of each phase. An example in the solid 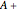 liquid region is given by:
liquid region is given by:
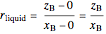 ,
,
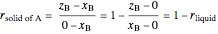 ,
,
where 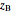 is the overall mole fraction of the mixture (the mole fraction of the point in the
is the overall mole fraction of the mixture (the mole fraction of the point in the 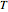 -
- -
-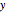 diagram),
diagram), 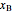 is the mole fraction of
is the mole fraction of 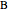 in the liquid phase, the mole fraction of
in the liquid phase, the mole fraction of 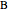 in the solid
in the solid 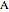 phase is zero,
phase is zero, 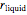 is the relative amount of liquid, and
is the relative amount of liquid, and 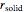 is the relative amount of solid
is the relative amount of solid 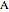 .
.
When the system is in solid-solid-liquid equilibrium, the relative amounts of each phase are found from mass balances. For example, using a value of 10.0% melted and an initial mole fraction of 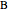 of 0.700 in the diagram:
of 0.700 in the diagram:
1. Determine the initial mole fraction of 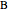 :
:
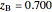
2. Determine the mole fraction of component 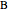 in each phase:
in each phase:
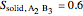
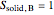

3. Set a basis for the amount of total moles in the system:
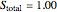
4. Determine the percentage melted:
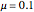
5. Determine the amount of liquid in the system:
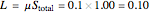
6. Perform a mole balance on the whole system:
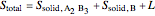
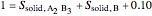
7. Perform a mole balance on component 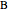 :
:
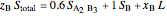
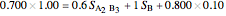
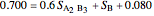
8. Simultaneously solve the equations 6 and 7 with the unknown variables (the number of equations should equal the number of unknown variables):
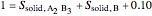
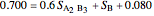
9. Relative amounts of each phase:
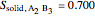 (relative amount of solid
(relative amount of solid 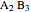 )
)
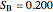 (relative amount of solid
(relative amount of solid 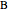 )
)
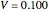 (relative amount of liquid)
(relative amount of liquid)
Permanent Citation