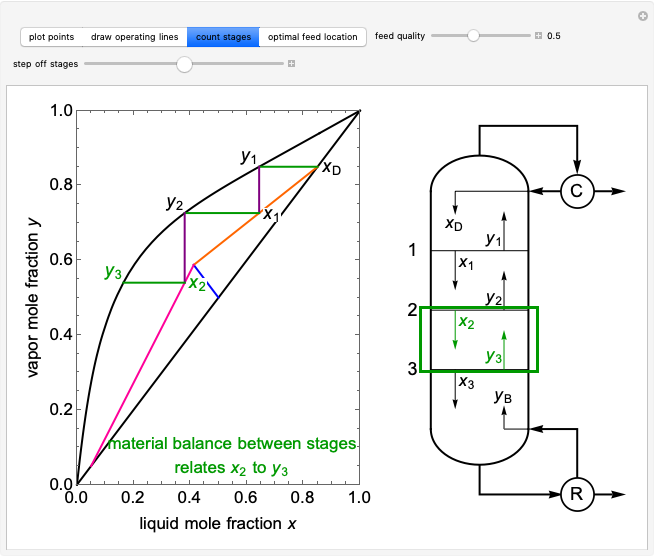
Adding One Component to a Binary Vapor-Liquid Equilibrium (VLE) Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
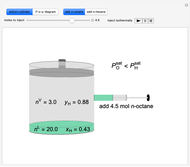
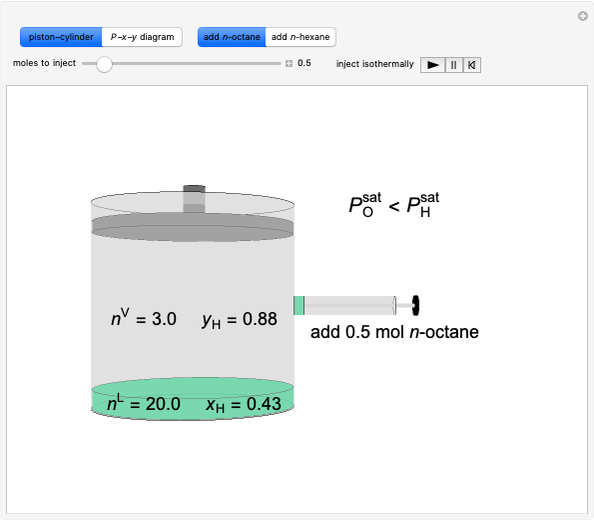
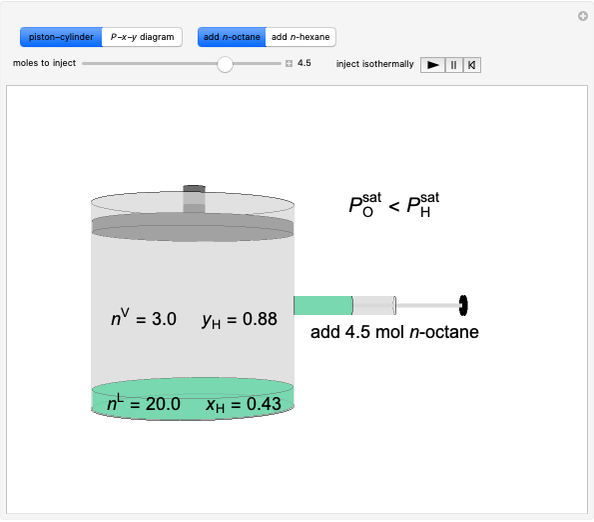
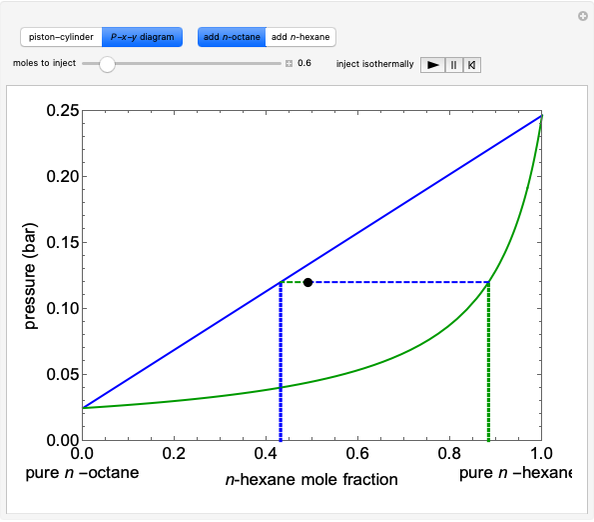
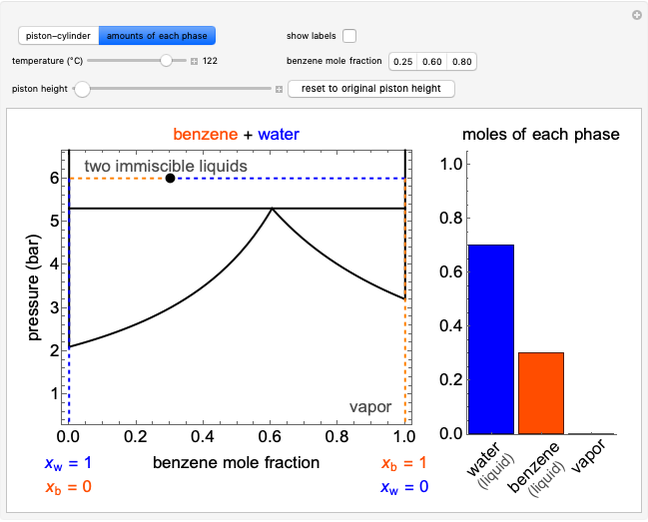
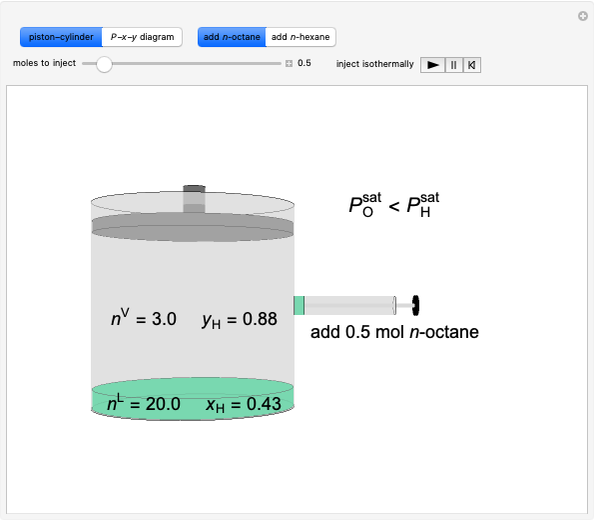
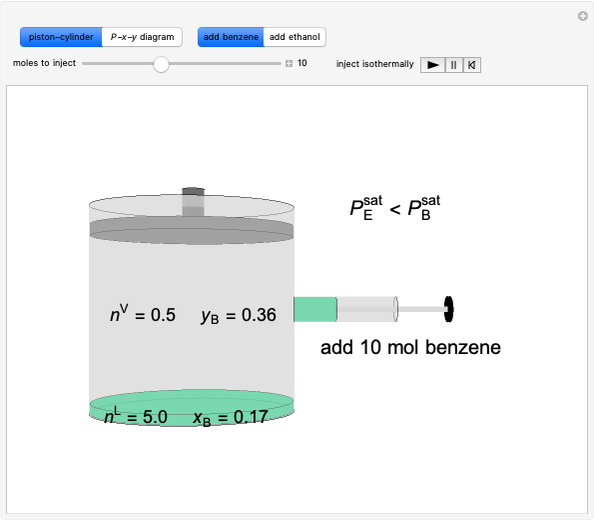
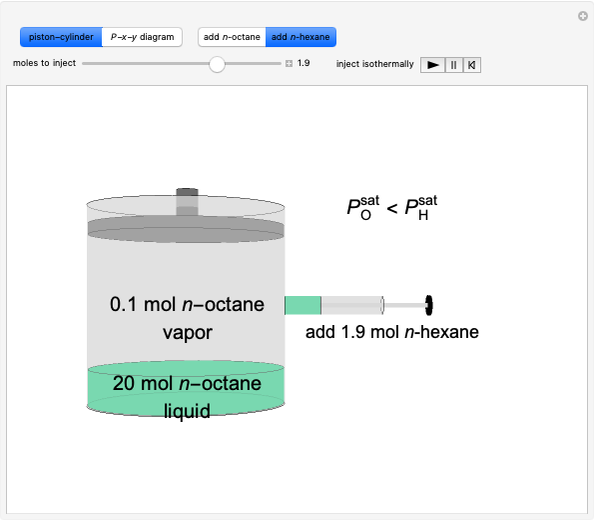
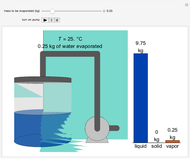
Initially an  -hexane/
-hexane/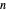 -octane mixture (20 mol of liquid, 3 mol of vapor) is in vapor-liquid equilibrium in a cylinder with a movable piston. Additional
-octane mixture (20 mol of liquid, 3 mol of vapor) is in vapor-liquid equilibrium in a cylinder with a movable piston. Additional 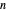 -hexane or
-hexane or 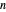 -octane is added when the inject isothermally play button is pressed. Change the number of moles injected at constant temperature and pressure with the slider. The system is modeled by Raoult’s law. The
-octane is added when the inject isothermally play button is pressed. Change the number of moles injected at constant temperature and pressure with the slider. The system is modeled by Raoult’s law. The 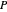 -
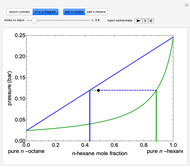
-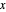 -
-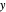 diagram shows what happens when additional alkane is injected. The moles in the liquid phase
diagram shows what happens when additional alkane is injected. The moles in the liquid phase 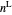 and the vapor phase
and the vapor phase 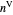 are displayed, as are the corresponding mole fractions of
are displayed, as are the corresponding mole fractions of 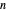 -hexane
-hexane 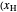 in liquid,
in liquid, 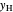 in vapor). Use the reset button to start the process again.
in vapor). Use the reset button to start the process again.
Contributed by: Rachael L. Baumann (March 2015)
Additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
This system is assumed to obey Raoult's law. The saturation pressures 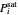 (bar) of
(bar) of 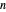 -hexane (
-hexane (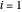 ) and
) and 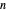 -octane (
-octane (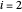 ) are calculated from the Antoine equation, where the Antoine coefficients
) are calculated from the Antoine equation, where the Antoine coefficients 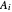 ,
, 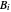 , and
, and 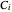 are constants and
are constants and 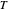 (°C) is temperature:
(°C) is temperature:
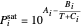 .
.
In order for the components to be in vapor-liquid equilibrium (VLE), the total pressure 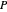 must equal the sum of the partial pressures:
must equal the sum of the partial pressures:
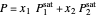 .
.
Here, 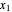 and
and 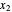 are the liquid mole fractions of
are the liquid mole fractions of 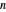 -hexane and
-hexane and 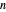 -octane. The mole fractions of
-octane. The mole fractions of 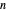 -hexane and
-hexane and 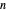 -octane in the vapor phase are
-octane in the vapor phase are 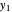 and
and 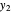 . Initially the system contains
. Initially the system contains 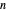 -hexane and
-hexane and 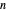 -octane in VLE. When
-octane in VLE. When 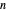 -hexane (the component with the higher vapor pressure) is added at constant temperature, vaporization returns the mixture to VLE. By contrast, if
-hexane (the component with the higher vapor pressure) is added at constant temperature, vaporization returns the mixture to VLE. By contrast, if 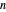 -octane (lower vapor pressure) is added at constant temperature, condensation returns the mixture to VLE.
-octane (lower vapor pressure) is added at constant temperature, condensation returns the mixture to VLE.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Adding One Component to a Binary VLE Mixture [Video]. (Dec 16, 2020) www.learncheme.com/simulations/thermodynamics/thermo-2/adding-one-component-to-a-binary-vle-mixture.
Permanent Citation