Arrhenius Equations for Reaction Rate and Viscosity

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
This Demonstration considers the effect of temperature on the rate of processes obeying the Arrhenius equation, specifically chemical reaction rates and fluid viscosity. We plot the reaction rate or viscosity as a function of the energy of activation. It is shown that increasing the temperature can accelerate chemical reaction rates and lower the viscosity of fluids.
Contributed by: Mark D. Normand, Maria G. Corradini, and Micha Peleg (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
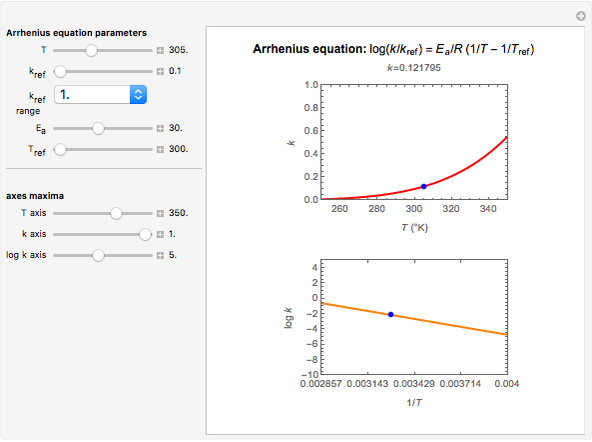
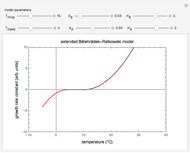
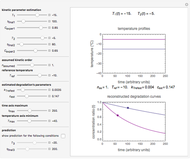
Snapshot 1: rate of reaction with a high energy of activation
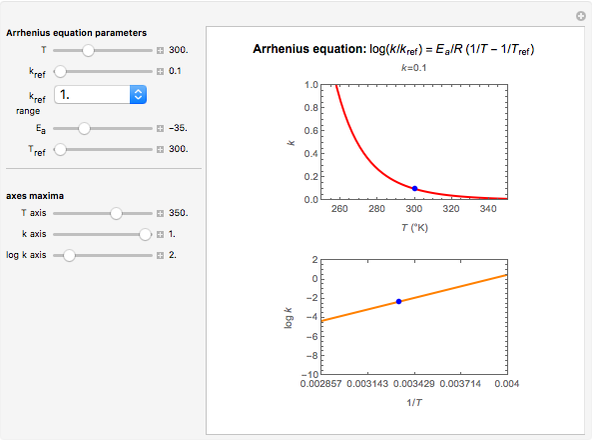
Snapshot 2: rate of reaction with a low energy of activation
Snapshot 3: effect of temperature on viscosity of a Newtonian fluid
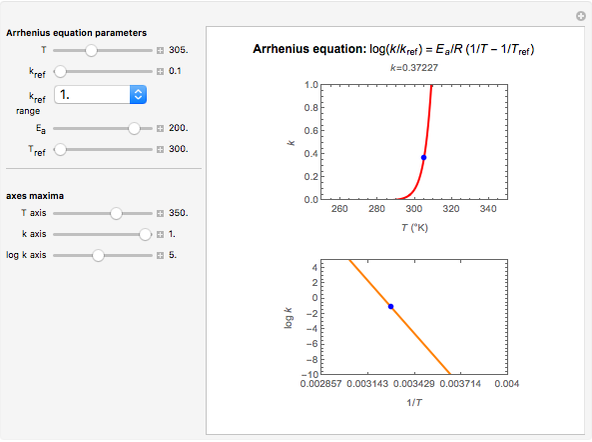
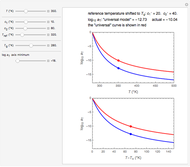
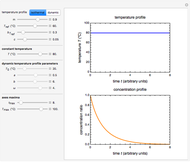
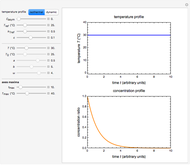
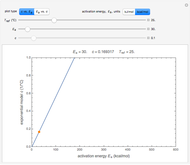
This Demonstration shows the temperature dependence of reaction rates or viscosity according to the Arrhenius equation, 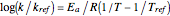 , where
, where 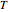 is the temperature,
is the temperature, 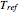 is a reference temperature (both in K),
is a reference temperature (both in K),  and
and 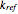 are appropriate parameter values at the respective temperatures,
are appropriate parameter values at the respective temperatures, 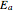 is the "energy of activation" (in J, kJ, cal, or kcal per mole), and
is the "energy of activation" (in J, kJ, cal, or kcal per mole), and 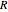 is the universal gas constant. If
is the universal gas constant. If 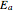 is positive, as in most chemical and biochemical reactions,
is positive, as in most chemical and biochemical reactions,  rises with temperature; if negative,
rises with temperature; if negative,  decreases with temperature, which is usually the case for viscosity.
decreases with temperature, which is usually the case for viscosity.
The Demonstration plots the  versus
versus 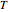 relationship on a linear scale (top), with the corresponding
relationship on a linear scale (top), with the corresponding 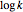 versus
versus 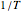 plot (bottom). The value of
plot (bottom). The value of  at the chosen temperature is shown as a blue dot on both plots and its numerical value is shown above the top plot.
at the chosen temperature is shown as a blue dot on both plots and its numerical value is shown above the top plot.
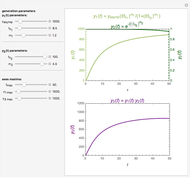
The values of the parameters can be entered directly or by moving the slider. The maximum value of the 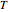 ,
,  , and
, and 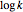 axes can also be adjusted. The order of magnitude of
axes can also be adjusted. The order of magnitude of 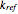 may be selected from a popup menu. Not all allowed settings produce plots that correspond to physical systems.
may be selected from a popup menu. Not all allowed settings produce plots that correspond to physical systems.
References:
D. Purich and R. D. Allison, Handbook of Biochemical Kinetics, San Diego, CA: Academic Press, 2000.
G. Drobny, P. Reid, and T. Engel, Physical Chemistry for the Life Sciences, Upper Saddle River, NJ: Prentice Hall, 2008.
Permanent Citation