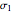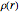Hydrides as Isoelectronic Perturbations of the Neon Atom

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
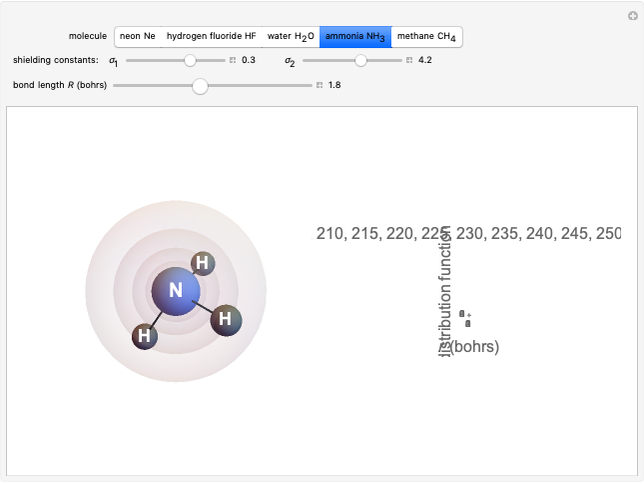
The second-row hydrides: hydrogen fluoride  , water
, water 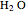 , ammonia
, ammonia 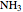 and methane
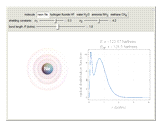
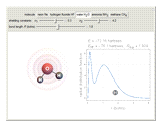
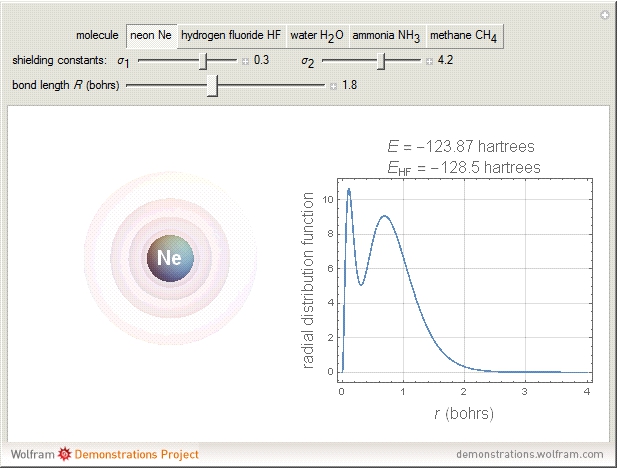
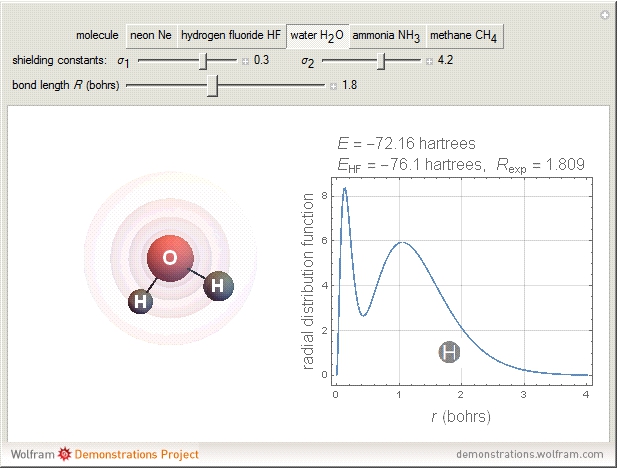
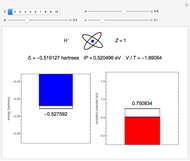
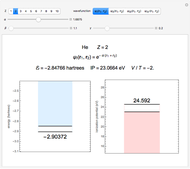
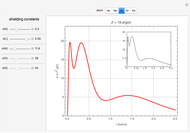
and methane  are isoelectronic with the Ne atom. The electronic structures for these 10-electron systems can be obtained, in concept, by perturbations on the Ne atom, in which protons are embedded in the electron cloud, while the central nuclear charge is reduced appropriately.
are isoelectronic with the Ne atom. The electronic structures for these 10-electron systems can be obtained, in concept, by perturbations on the Ne atom, in which protons are embedded in the electron cloud, while the central nuclear charge is reduced appropriately.
Contributed by: S. M. Blinder (July 2019)
Open content licensed under CC BY-NC-SA
Details
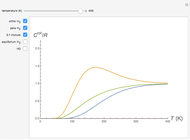
The 10-electron density function is approximated by
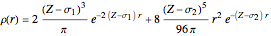 ,
,
following [1]. The resulting density functional contains the usual forms for kinetic and potential energies, plus the additional contributions from the hydrogen atoms in  ,
,  ,
, 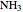 and
and 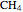 .
.
Experimental values of parameters: 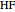 :
:  ;
; 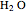 :
:  ,
,  ;
; 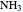 :
: 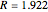 ,
, 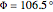 ;
; 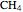 :
:  ,
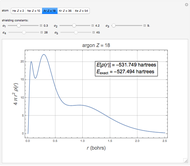
, 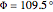 . Approximate Hartree–Fock energies (hartrees):
. Approximate Hartree–Fock energies (hartrees): 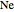 :
: 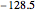 ,
, 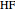 :
: 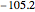 ,
, 
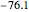 ,
, 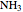 :
:  ,
, 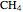 :
: 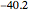 .
.
Reference
[1] S. M. Blinder. "Density Functional Computations on Noble Gas Atoms" from the Wolfram Demonstrations Project—A Wolfram Web Resource. demonstrations.wolfram.com/DensityFunctionalComputationsOnNobleGasAtoms.
Snapshots
Permanent Citation