Ions with Noble Gas Configurations

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
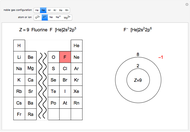
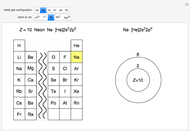
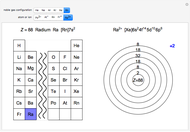
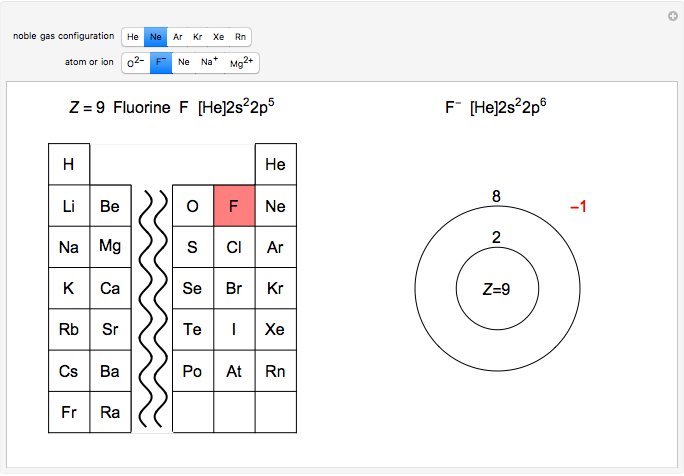
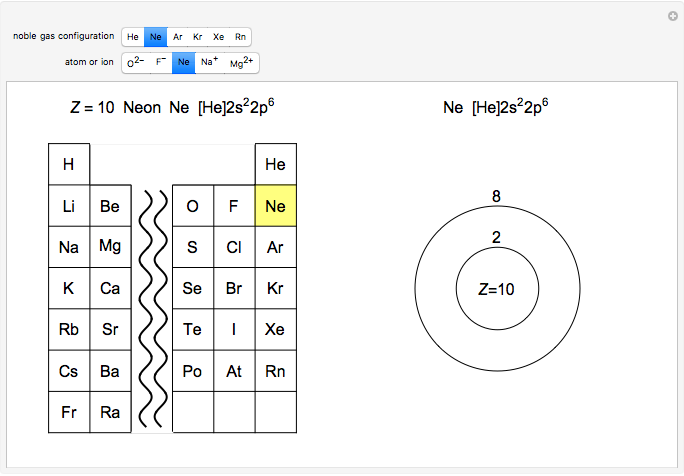
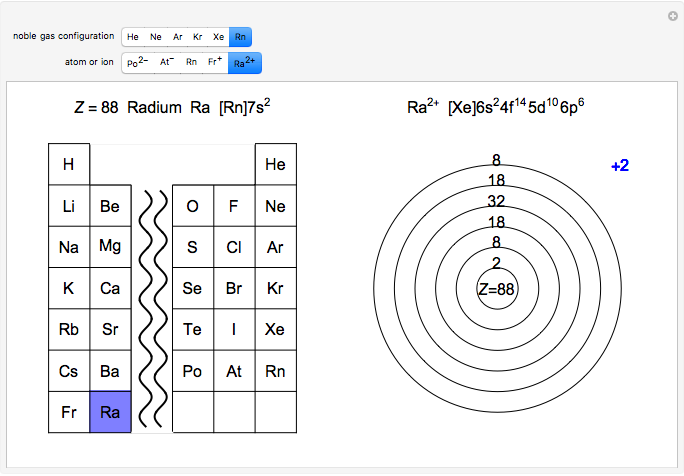
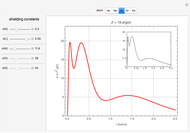
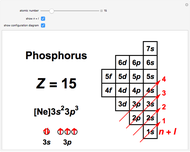
Atoms and atomic ions with sequences of completely filled electron shells exhibit enhanced stability. The prime examples are the noble gases, He, Ne, Ar, Kr, Xe, and Rn, containing one of the "magic numbers" of electrons: 2, 10, 18, 36, 54, and 86, respectively. These gases are colorless, odorless, and chemically inert (although a few compounds of Kr, Xe, and Rn have been synthesized in recent years).
[more]
Contributed by: S. M. Blinder (May 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Ions with Noble Gas Configurations"
http://demonstrations.wolfram.com/IonsWithNobleGasConfigurations/
Wolfram Demonstrations Project
Published: May 28 2013