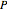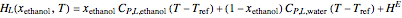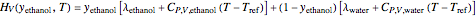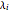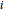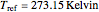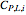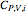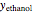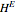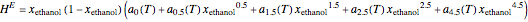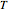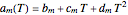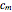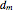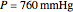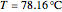Ponchon-Savarit Diagram for an Ethanol-Water Binary Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
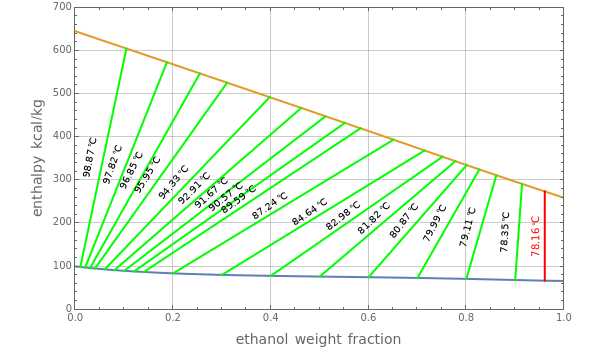
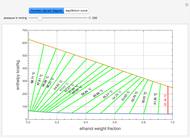
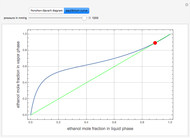
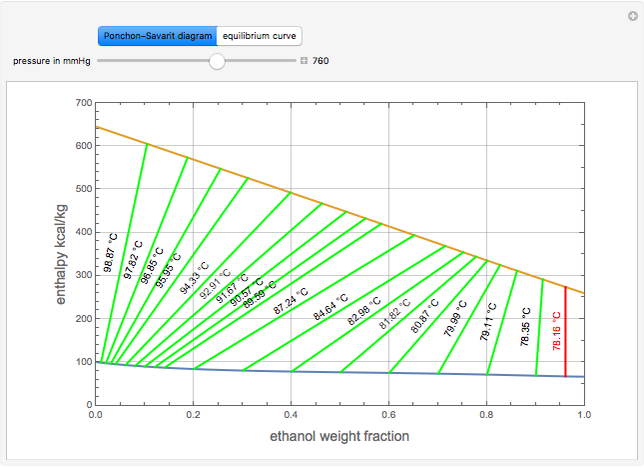
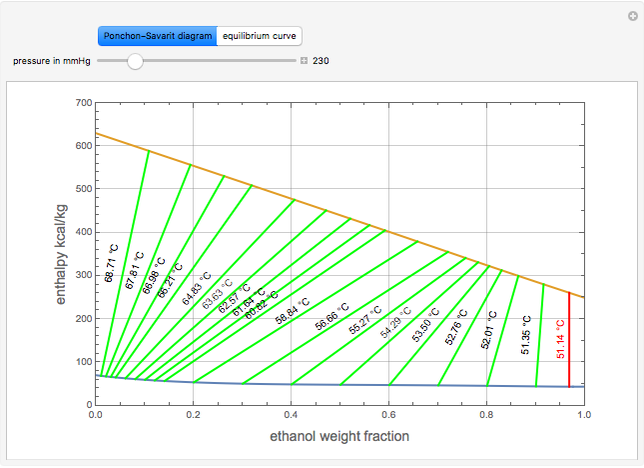
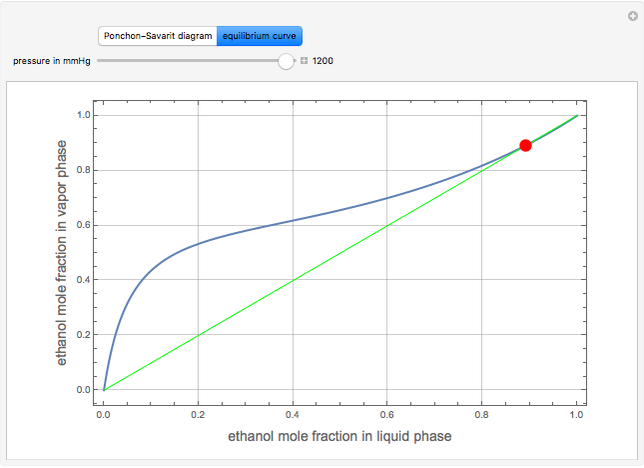
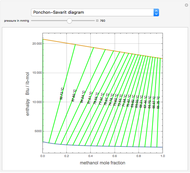
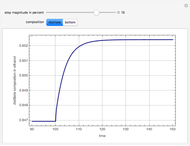
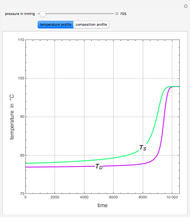
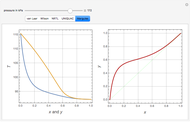
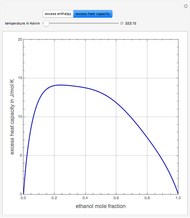
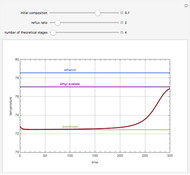
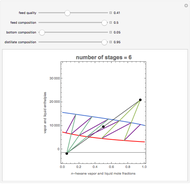
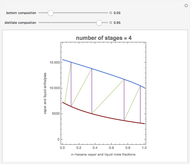
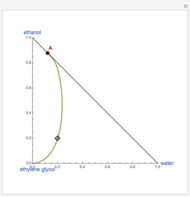
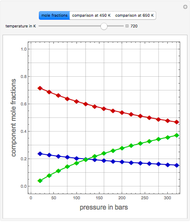
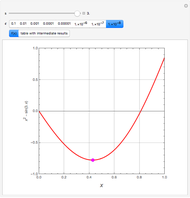
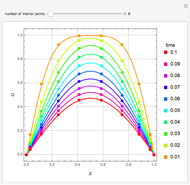
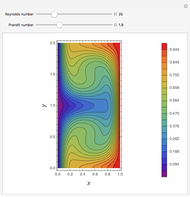
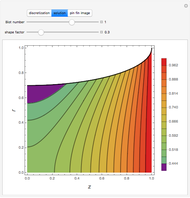
The Ponchon–Savarit diagram provides a graphical method for solving coupled material and energy balances in separation processes involving binary mixtures. This Demonstration illustrates how the Ponchon–Savarit diagram can be computed for a nonideal binary mixture that has an azeotrope.
[more]
Contributed by: Housam Binous, Nadhir A. Al-Baghli, and Brian G. Higgins (January 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] P. C. Wankat, Separation Process Engineering, 3rd ed., Upper Saddle River, NJ: Pearson, 2012.
[2] J. A. Larkin, "Thermodynamic Properties of Aqueous Non-Electrolyte Mixtures I. Excess Enthalpy for Water + Ethanol at 298.15 to 383.15 K," Journal of Chemical Thermodynamics, 7(2), 1975 pp. 137–148.
[3] R. H. Perry and D. W. Green, Perry's Chemical Engineers' Handbook, 7th ed., New York: McGraw–Hill, 1997.
Permanent Citation